Abstract
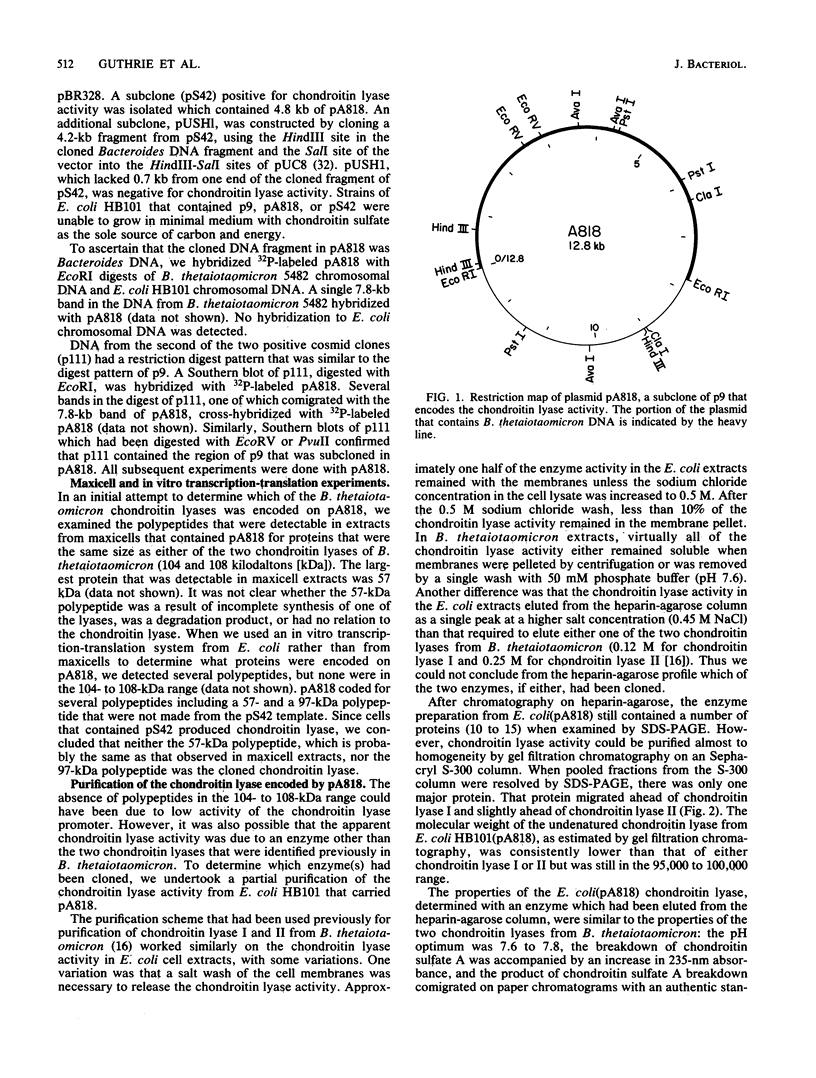
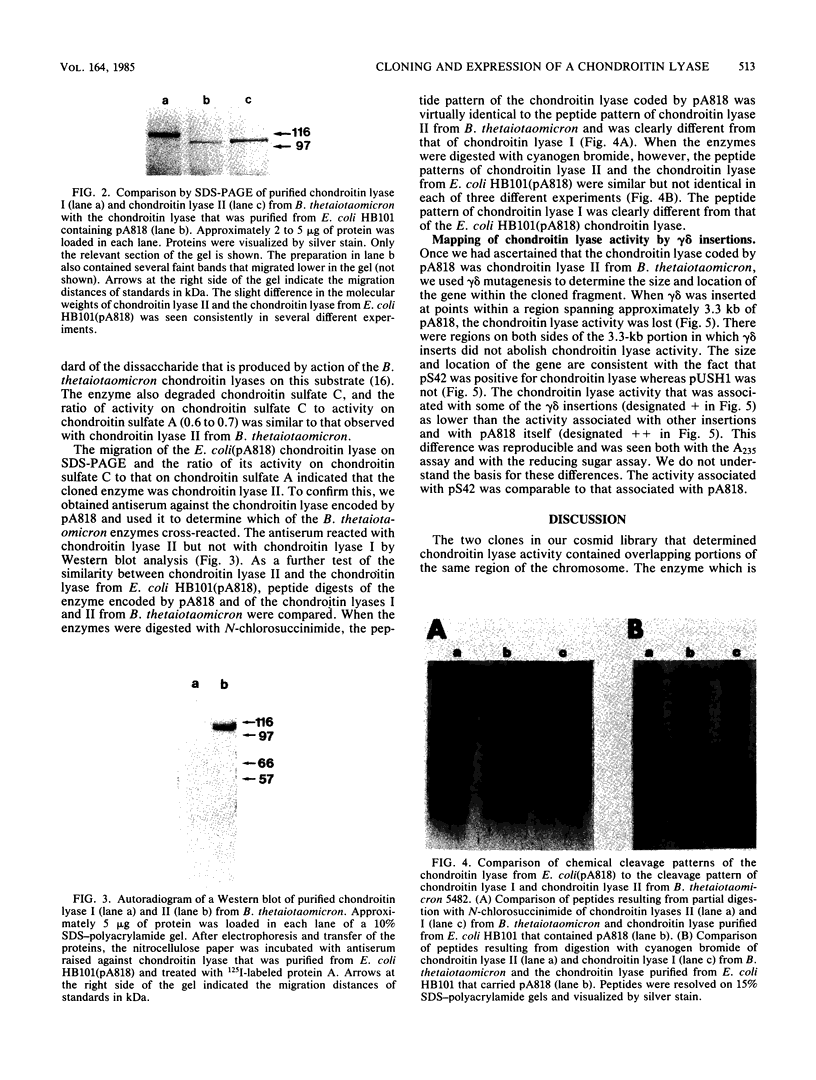
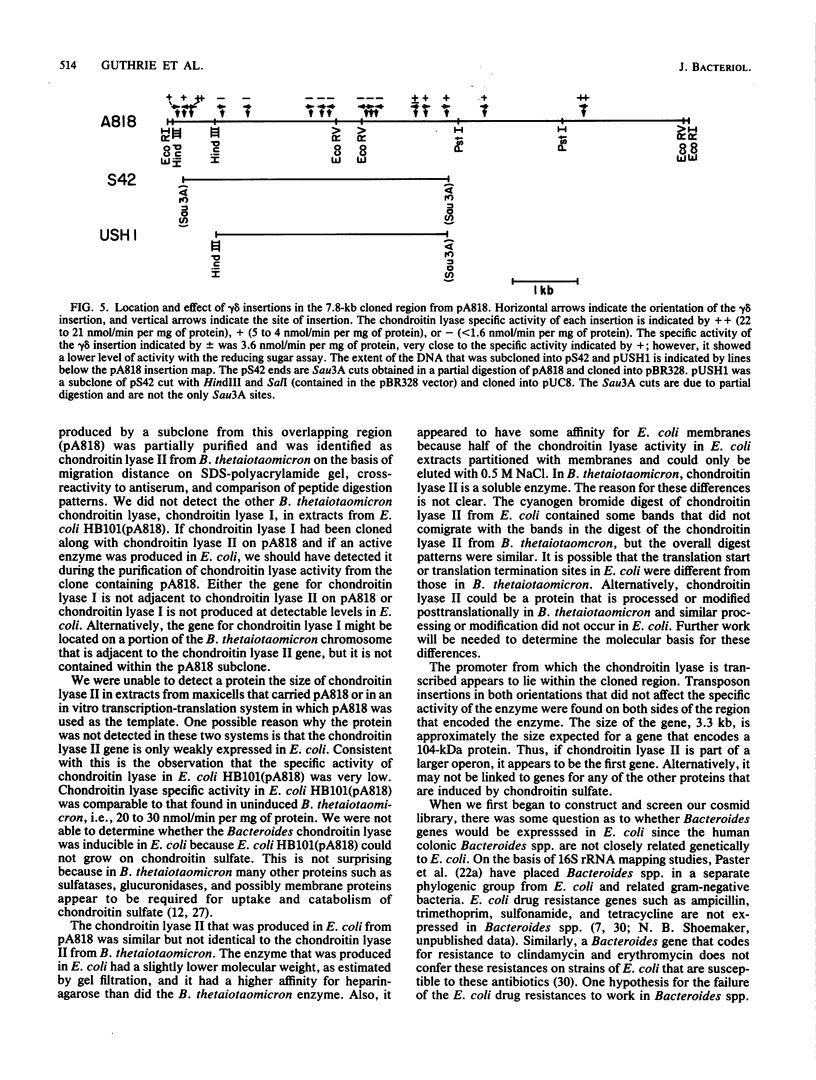
We cloned the gene for one of the two chondroitin lyases of Bacteroides thetaiotaomicron into the cosmid vector pHC79 and subcloned it into pBR328. No proteins the size of B. thetaiotaomicron chondroitin lyase I or II (104 to 108 kilodaltons) were detectable in maxicell or in vitro transcription-translation preparations. However, partial purification of the chondroitin lyase activity from the Escherichia coli subclone showed that its properties were similar to those of the B. thetaiotaomicron chondroitin lyases. Antibodies to the chondroitin lyase that was produced in E. coli cross-reacted with the B. thetaiotaomicron chondroitin lyase II but not with chondroitin lyase I. The molecular weight of the enzyme produced in E. coli, as measured by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and by gel filtration, was slightly lower than those of the two chondroitin lyases from B. thetaiotaomicron; the enzyme had a higher affinity for bacterial membranes and for heparin-agarose, and cyanogen bromide digestion products of the chondroitin lyase produced in E. coli differed slightly from those of B. thetaiotaomicron chondroitin lyase II. gamma delta mutagenesis was used to locate the chondroitin lyase gene on the subcloned 7.8-kilobase EcoRI fragment. The size of the gene was approximately 3.3 kilobases, as expected for a protein with a molecular weight of 104,000.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson B. J., Bills M. M., Egerton J. R., Mattick J. S. Cloning and expression in Escherichia coli of the gene encoding the structural subunit of Bacteroides nodosus fimbriae. J Bacteriol. 1984 Nov;160(2):748–754. doi: 10.1128/jb.160.2.748-754.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chory J., Kaplan S. The in vitro transcription-translation of DNA and RNA templates by extracts of Rhodopseudomonas sphaeroides. Optimization and comparison of template specificity with Escherichia coli extracts and in vivo synthesis. J Biol Chem. 1982 Dec 25;257(24):15110–15121. [PubMed] [Google Scholar]
- Dygert S., Li L. H., Florida D., Thoma J. A. Determination of reducing sugar with improved precision. Anal Biochem. 1965 Dec;13(3):367–374. doi: 10.1016/0003-2697(65)90327-1. [DOI] [PubMed] [Google Scholar]
- Erickson P. F., Minier L. N., Lasher R. S. Quantitative electrophoretic transfer of polypeptides from SDS polyacrylamide gels to nitrocellulose sheets: a method for their re-use in immunoautoradiographic detection of antigens. J Immunol Methods. 1982 Jun 11;51(2):241–249. doi: 10.1016/0022-1759(82)90263-0. [DOI] [PubMed] [Google Scholar]
- Eschenbruch M., Bürk R. R. Experimentally improved reliability of ultrasensitive silver staining of protein in polyacrylamide gels. Anal Biochem. 1982 Sep 1;125(1):96–99. doi: 10.1016/0003-2697(82)90387-6. [DOI] [PubMed] [Google Scholar]
- Guiney D. G., Hasegawa P., Davis C. E. Plasmid transfer from Escherichia coli to Bacteroides fragilis: differential expression of antibiotic resistance phenotypes. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7203–7206. doi: 10.1073/pnas.81.22.7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer M. S. The gamma delta sequence of F is an insertion sequence. J Mol Biol. 1978 Dec 15;126(3):347–365. doi: 10.1016/0022-2836(78)90045-1. [DOI] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Kotarski S. F., Salyers A. A. Isolation and characterization of outer membranes of Bacteroides thetaiotaomicron grown on different carbohydrates. J Bacteriol. 1984 Apr;158(1):102–109. doi: 10.1128/jb.158.1.102-109.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz R. G., Gennis R. B. A quantitative radioimmunological screening method for specific gene products. Anal Biochem. 1982 Dec;127(2):247–257. doi: 10.1016/0003-2697(82)90169-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn S., Chan T., Lipeski L., Salyers A. A. Isolation and characterization of two chondroitin lyases from Bacteroides thetaiotaomicron. J Bacteriol. 1983 Nov;156(2):859–866. doi: 10.1128/jb.156.2.859-866.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lischwe M. A., Ochs D. A new method for partial peptide mapping using N-chlorosuccinimide/urea and peptide silver staining in sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem. 1982 Dec;127(2):453–457. doi: 10.1016/0003-2697(82)90203-2. [DOI] [PubMed] [Google Scholar]
- Lopatin D. E., Voss E. W., Jr Anti-lysergyl antibody: measurement of binding parameters in IgG fractions. Immunochemistry. 1974 Jun;11(6):285–293. doi: 10.1016/0019-2791(74)90364-4. [DOI] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Microbiol. 1974 May;27(5):961–979. doi: 10.1128/am.27.5.961-979.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikodem V., Fresco J. R. Protein fingerprinting by SDS-gel electrophoresis after partial fragmentation with CNBr. Anal Biochem. 1979 Sep 1;97(2):382–386. doi: 10.1016/0003-2697(79)90089-7. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Salyers A. A., Kotarski S. F. Induction of chondroitin sulfate lyase activity in Bacteroides thetaiotaomicron. J Bacteriol. 1980 Aug;143(2):781–788. doi: 10.1128/jb.143.2.781-788.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., O'Brien M. Cellular location of enzymes involved in chondroitin sulfate breakdown by Bacteroides thetaiotaomicron. J Bacteriol. 1980 Aug;143(2):772–780. doi: 10.1128/jb.143.2.772-780.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., Vercellotti J. R., West S. E., Wilkins T. D. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl Environ Microbiol. 1977 Feb;33(2):319–322. doi: 10.1128/aem.33.2.319-322.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Guthrie E. P., Salyers A. A., Gardner J. F. Evidence that the clindamycin-erythromycin resistance gene of Bacteroides plasmid pBF4 is on a transposable element. J Bacteriol. 1985 May;162(2):626–632. doi: 10.1128/jb.162.2.626-632.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]