Abstract
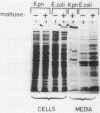
Some strains of Klebsiella pneumonia secrete pullulanase, a debranching enzyme which produces linear molecules (maltodextrins, amylose) from amylopectin and glycogen. pulA, the structural gene for pullulanase, was introduced into Escherichia coli, either on a multiple-copy-number plasmid or as a single copy in the chromosome. When in E. coli, pulA was controlled by malT, the positive regulatory gene of the maltose regulon. Indeed, pulA expression was undetectable in a malT-negative mutant and constitutive in a malTc strain. Furthermore, the plasmid carrying pulA titrated the MalT protein. When produced in E. coli, pullulanase was not localized in the same way as in K. pneumoniae. In the latter case it was first exported to the outer membrane, with which it remained loosely associated, and was then released into the growth medium. In E. coli the enzyme was distributed both in the inner and the outer membranes and was never released into the growth medium.
Full text
PDF


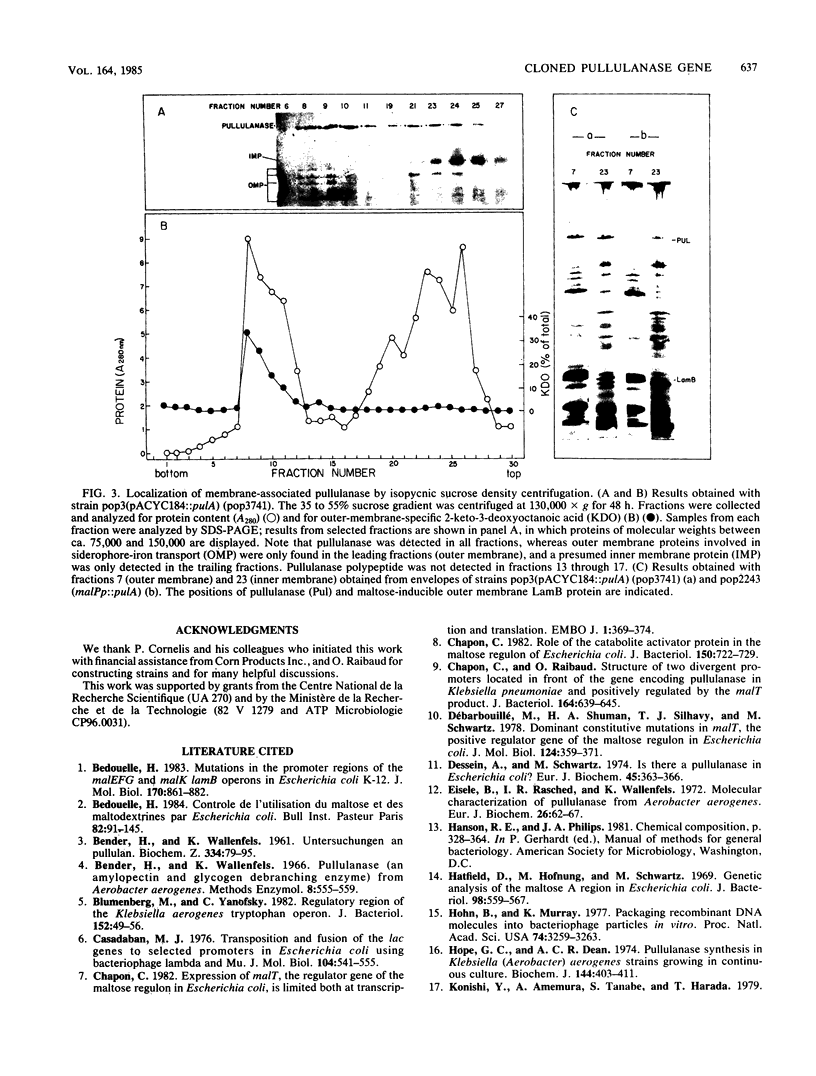

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedouelle H. Mutations in the promoter regions of the malEFG and malK-lamB operons of Escherichia coli K12. J Mol Biol. 1983 Nov 15;170(4):861–882. doi: 10.1016/s0022-2836(83)80192-2. [DOI] [PubMed] [Google Scholar]
- Blumenberg M., Yanofsky C. Regulatory region of the Klebsiella aerogenes tryptophan operon. J Bacteriol. 1982 Oct;152(1):49–56. doi: 10.1128/jb.152.1.49-56.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Chapon C. Expression of malT, the regulator gene of the maltose region in Escherichia coli, is limited both at transcription and translation. EMBO J. 1982;1(3):369–374. doi: 10.1002/j.1460-2075.1982.tb01176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapon C. Role of the catabolite activator protein in the maltose regulon of Escherichia coli. J Bacteriol. 1982 May;150(2):722–729. doi: 10.1128/jb.150.2.722-729.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessein A., Schwartz M. Is there a pullulanase in escherichia coli? Eur J Biochem. 1974 Jun 15;45(2):363–366. doi: 10.1111/j.1432-1033.1974.tb03561.x. [DOI] [PubMed] [Google Scholar]
- Débarbouillé M., Shuman H. A., Silhavy T. J., Schwartz M. Dominant constitutive mutations in malT, the positive regulator gene of the maltose regulon in Escherichia coli. J Mol Biol. 1978 Sep 15;124(2):359–371. doi: 10.1016/0022-2836(78)90304-2. [DOI] [PubMed] [Google Scholar]
- Eisele B., Rasched I. R., Wallenfels K. Molecular characterization of pullulanase from Aerobacter aerogenes. Eur J Biochem. 1972 Mar 15;26(1):62–67. doi: 10.1111/j.1432-1033.1972.tb01739.x. [DOI] [PubMed] [Google Scholar]
- Hatfield D., Hofnung M., Schwartz M. Genetic analysis of the maltose A region in Escherichia coli. J Bacteriol. 1969 May;98(2):559–567. doi: 10.1128/jb.98.2.559-567.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Murray K. Packaging recombinant DNA molecules into bacteriophage particles in vitro. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3259–3263. doi: 10.1073/pnas.74.8.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope G. C., Dean A. C. Pullulanase synthesis in klebsiella (aerobacter) aerogenes strains growing in continuous culture. Biochem J. 1974 Nov;144(2):403–411. doi: 10.1042/bj1440403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Murray N. E., Brammar W. J., Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977 Jan 7;150(1):53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Schnaitman C. A. Factors affecting the electrophoretic mobility of the major outer membrane proteins of Escherichia coli in polyacrylamide gels. Biochim Biophys Acta. 1979 Nov 23;581(1):163–178. doi: 10.1016/0005-2795(79)90233-2. [DOI] [PubMed] [Google Scholar]
- Raibaud O., Débarbouillé M., Schwartz M. Use of deletions created in vitro to map transcriptional regulatory signals in the malA region of Escherichia coli. J Mol Biol. 1983 Jan 25;163(3):395–408. doi: 10.1016/0022-2836(83)90065-7. [DOI] [PubMed] [Google Scholar]
- Raibaud O., Gutierrez C., Schwartz M. Essential and nonessential sequences in malPp, a positively controlled promoter in Escherichia coli. J Bacteriol. 1985 Mar;161(3):1201–1208. doi: 10.1128/jb.161.3.1201-1208.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raibaud O., Mock M., Schwartz M. A technique for integrating any DNA fragment into the chromosome of Escherichia coli. Gene. 1984 Jul-Aug;29(1-2):231–241. doi: 10.1016/0378-1119(84)90183-5. [DOI] [PubMed] [Google Scholar]
- Reeve J. Use of minicells for bacteriophage-directed polypeptide synthesis. Methods Enzymol. 1979;68:493–503. doi: 10.1016/0076-6879(79)68038-2. [DOI] [PubMed] [Google Scholar]
- Wallenfels K., Bender H., Rached J. R. Pullulanase from Aerobacter aerogenes; production in a cell-bound state. Purification and properties of the enzyme. Biochem Biophys Res Commun. 1966 Feb 3;22(3):254–261. doi: 10.1016/0006-291x(66)90474-8. [DOI] [PubMed] [Google Scholar]
- Wandersman C., Schwartz M., Ferenci T. Escherichia coli mutants impaired in maltodextrin transport. J Bacteriol. 1979 Oct;140(1):1–13. doi: 10.1128/jb.140.1.1-13.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöhner G., Wöber G. Pullulanase, an enzyme of starch catabolism, is associated with the outer membrane of Klebsiella. Arch Microbiol. 1978 Mar;116(3):303–310. doi: 10.1007/BF00417856. [DOI] [PubMed] [Google Scholar]