Abstract
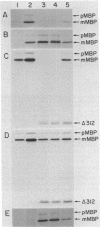
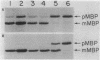
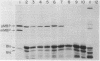
It has not been possible to obtain in vitro expression of the positively regulated malE gene encoding the periplasmic maltose-binding protein (MBP) of Escherichia coli. To facilitate in vitro malE expression, we constructed plasmids that place the malE gene under transcriptional control of the lacUV5 promoter-operator. These plasmids could be grouped into three classes, based upon their ability to complement in vivo a chromosomal malE deletion in the presence or absence of isopropyl thiogalactoside. In the one class I plasmid analyzed, the lacUV5-malE junction was just 3' to the malE ATG initiation codon, and this plasmid did not complement the malE deletion. Class II and class III plasmids retained various amounts of the malE promoter. MBP synthesis was solely under control of the lacUV5 promoter in the class II plasmids, and MBP synthesis was under control of both the lacUV5 and malE promoters in the class III plasmids. A malE mutation that renders the MBP signal peptide export defective was genetically recombined onto one of the class II plasmids. The in vivo synthesis and export of plasmid-encoded MBP were studied in the presence and absence of isopropyl thiogalactoside and maltose and in a strain harboring a prlA mutation that suppresses the malE signal sequence mutation and is thought to alter the export machinery of cells. In addition, both class II and class III plasmids programmed the synthesis of precursor MBP in an in vitro-coupled transcription-translation system. When precursor MBP was synthesized in vitro in the presence of E. coli membrane vesicles, a significant portion of wild-type precursor MBP, but not export-defective precursor MBP, was converted to a form that migrated on sodium dodecyl sulfate-polyacrylamide gels identically to mature MBP synthesized in vivo.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bankaitis V. A., Bassford P. J., Jr Proper interaction between at least two components is required for efficient export of proteins to the Escherichia coli cell envelope. J Bacteriol. 1985 Jan;161(1):169–178. doi: 10.1128/jb.161.1.169-178.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankaitis V. A., Rasmussen B. A., Bassford P. J., Jr Intragenic suppressor mutations that restore export of maltose binding protein with a truncated signal peptide. Cell. 1984 May;37(1):243–252. doi: 10.1016/0092-8674(84)90320-9. [DOI] [PubMed] [Google Scholar]
- Bassford P., Beckwith J. Escherichia coli mutants accumulating the precursor of a secreted protein in the cytoplasm. Nature. 1979 Feb 15;277(5697):538–541. doi: 10.1038/277538a0. [DOI] [PubMed] [Google Scholar]
- Bedouelle H., Bassford P. J., Jr, Fowler A. V., Zabin I., Beckwith J., Hofnung M. Mutations which alter the function of the signal sequence of the maltose binding protein of Escherichia coli. Nature. 1980 May 8;285(5760):78–81. doi: 10.1038/285078a0. [DOI] [PubMed] [Google Scholar]
- Bedouelle H., Hofnung M. A DNA sequence containing the control regions of the malEFG and malK-lamB operons in Escherichia coli K12. Mol Gen Genet. 1982;185(1):82–87. doi: 10.1007/BF00333794. [DOI] [PubMed] [Google Scholar]
- Bedouelle H. Mutations in the promoter regions of the malEFG and malK-lamB operons of Escherichia coli K12. J Mol Biol. 1983 Nov 15;170(4):861–882. doi: 10.1016/s0022-2836(83)80192-2. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Chen L., Rhoads D., Tai P. C. Alkaline phosphatase and OmpA protein can be translocated posttranslationally into membrane vesicles of Escherichia coli. J Bacteriol. 1985 Mar;161(3):973–980. doi: 10.1128/jb.161.3.973-980.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debarbouille M., Schwartz M. The use of gene fusions to study the expression of malT the positive regulator gene of the maltose regulon. J Mol Biol. 1979 Aug 15;132(3):521–534. doi: 10.1016/0022-2836(79)90273-0. [DOI] [PubMed] [Google Scholar]
- DiRienzo J. M., Nakamura K., Inouye M. The outer membrane proteins of Gram-negative bacteria: biosynthesis, assembly, and functions. Annu Rev Biochem. 1978;47:481–532. doi: 10.1146/annurev.bi.47.070178.002405. [DOI] [PubMed] [Google Scholar]
- Duplay P., Bedouelle H., Fowler A., Zabin I., Saurin W., Hofnung M. Sequences of the malE gene and of its product, the maltose-binding protein of Escherichia coli K12. J Biol Chem. 1984 Aug 25;259(16):10606–10613. [PubMed] [Google Scholar]
- Emr S. D., Bassford P. J., Jr Localization and processing of outer membrane and periplasmic proteins in Escherichia coli strains harboring export-specific suppressor mutations. J Biol Chem. 1982 May 25;257(10):5852–5860. [PubMed] [Google Scholar]
- Emr S. D., Hanley-Way S., Silhavy T. J. Suppressor mutations that restore export of a protein with a defective signal sequence. Cell. 1981 Jan;23(1):79–88. doi: 10.1016/0092-8674(81)90272-5. [DOI] [PubMed] [Google Scholar]
- Ferro-Novick S., Honma M., Beckwith J. The product of gene secC is involved in the synthesis of exported proteins in E. coli. Cell. 1984 Aug;38(1):211–217. doi: 10.1016/0092-8674(84)90542-7. [DOI] [PubMed] [Google Scholar]
- Josefsson L. G., Randall L. L. Processing in vivo of precursor maltose-binding protein in Escherichia coli occurs post-translationally as well as co-translationally. J Biol Chem. 1981 Mar 10;256(5):2504–2507. [PubMed] [Google Scholar]
- Kellermann O., Szmelcman S. Active transport of maltose in Escherichia coli K12. Involvement of a "periplasmic" maltose binding protein. Eur J Biochem. 1974 Aug 15;47(1):139–149. doi: 10.1111/j.1432-1033.1974.tb03677.x. [DOI] [PubMed] [Google Scholar]
- Lauer G., Pastrana R., Sherley J., Ptashne M. Construction of overproducers of the bacteriophage 434 repressor and cro proteins. J Mol Appl Genet. 1981;1(2):139–147. [PubMed] [Google Scholar]
- Marchal C., Greenblatt J., Hofnung M. malB region in Escherichia coli K-12: specialized transducing bacteriophages and first restriction map. J Bacteriol. 1978 Dec;136(3):1109–1119. doi: 10.1128/jb.136.3.1109-1119.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchal C., Perrin D., Hedgpeth J., Hofnung M. Synthesis and maturation of lambda receptor in Escherichia coli K-12: in vivo and in vitro expression of gene lamB under lac promoter control. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1491–1495. doi: 10.1073/pnas.77.3.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Model P., Russel M. Insertion of proteins into bacterial membranes. Methods Enzymol. 1983;97:138–146. doi: 10.1016/0076-6879(83)97127-6. [DOI] [PubMed] [Google Scholar]
- Müller M., Blobel G. In vitro translocation of bacterial proteins across the plasma membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7421–7425. doi: 10.1073/pnas.81.23.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. Regulation of a membrane component required for protein secretion in Escherichia coli. Cell. 1982 Aug;30(1):311–319. doi: 10.1016/0092-8674(82)90037-x. [DOI] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J., Josefsson L. G. Precursors of three exported proteins in Escherichia coli. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1209–1212. doi: 10.1073/pnas.75.3.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J. Synthesis of exported proteins by membrane-bound polysomes from Escherichia coli. Eur J Biochem. 1977 May 2;75(1):43–53. doi: 10.1111/j.1432-1033.1977.tb11502.x. [DOI] [PubMed] [Google Scholar]
- Randall L. L. Translocation of domains of nascent periplasmic proteins across the cytoplasmic membrane is independent of elongation. Cell. 1983 May;33(1):231–240. doi: 10.1016/0092-8674(83)90352-5. [DOI] [PubMed] [Google Scholar]
- Rasmussen B. A., Bassford P. J., Jr Both linked and unlinked mutations can alter the intracellular site of synthesis of exported proteins of Escherichia coli. J Bacteriol. 1985 Jan;161(1):258–264. doi: 10.1128/jb.161.1.258-264.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy T. J., Benson S. A., Emr S. D. Mechanisms of protein localization. Microbiol Rev. 1983 Sep;47(3):313–344. doi: 10.1128/mr.47.3.313-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]