Abstract
The plasmid pCD1 is required for expression of the low-calcium response (LCR), virulence, and production of V antigen in Yersinia pestis KIM. Five independent mutants constitutive for the LCR at 37 degrees C (Lcrc) were obtained through ethyl methanesulfonate mutagenesis followed by ampicillin enrichment. A sixth, spontaneous mutant was obtained directly through ampicillin enrichment. These mutants failed to grow at 37 degrees C regardless of calcium concentration and produced V antigen constitutively at this temperature. All six mutations were located on pCD1. One mutation was mapped to a 1-kilobase region of lcrA. Based on complementation mapping of this mutation, the lcrA locus was divided into two new loci, lcrD and lcrE. This mutation, lcrE1, did not alter the transcription of other genes in the LCR region and was cis-recessive to lcr mutations. Several lower-molecular-weight outer membrane proteins which were observed in the parent strain grown at 37 degrees C in the presence of 2.5 mM calcium were reduced in quantity or absent from the mutant strain.
Full text
PDF



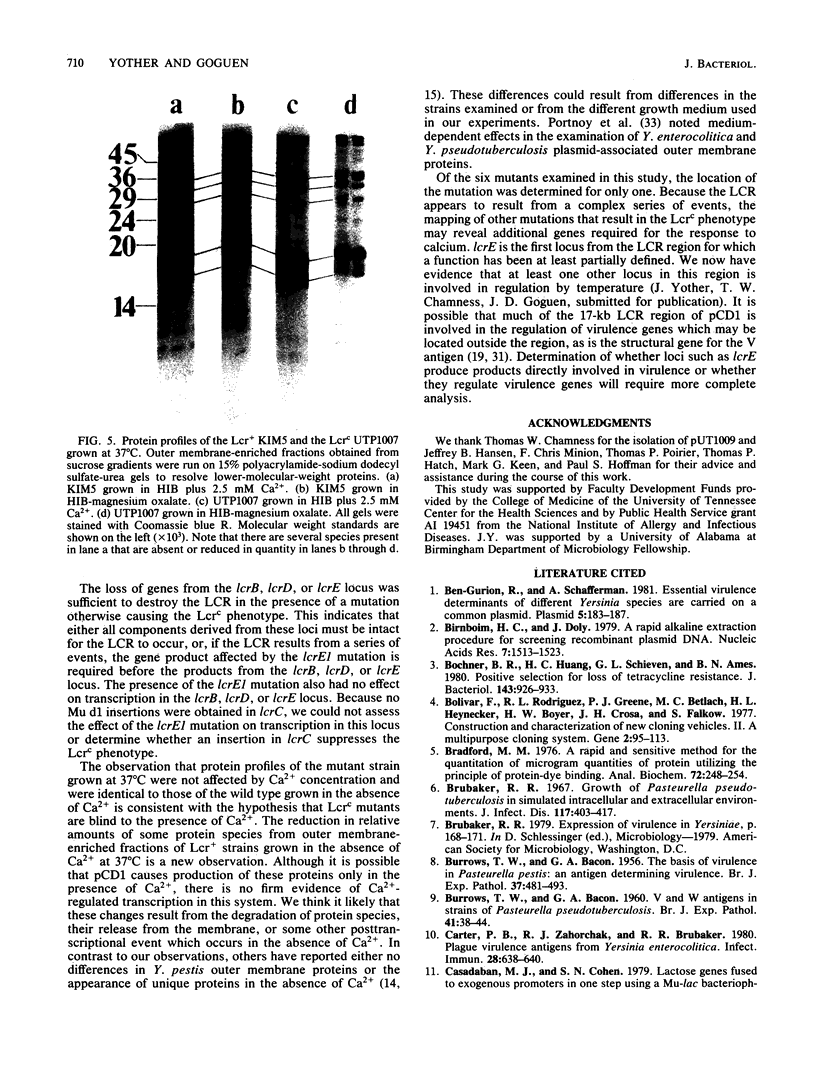

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACON G. A., BURROWS T. W. The basis of virulence in Pasteurella pestis: an antigen determining virulence. Br J Exp Pathol. 1956 Oct;37(5):481–493. [PMC free article] [PubMed] [Google Scholar]
- BURROWS T. W., BACON G. A. V and W antigens in strains of Pasteurella pseudotuberculosis. Br J Exp Pathol. 1960 Feb;41:38–44. [PMC free article] [PubMed] [Google Scholar]
- Ben-Gurion R., Shafferman A. Essential virulence determinants of different Yersinia species are carried on a common plasmid. Plasmid. 1981 Mar;5(2):183–187. doi: 10.1016/0147-619x(81)90019-6. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner B. R., Huang H. C., Schieven G. L., Ames B. N. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980 Aug;143(2):926–933. doi: 10.1128/jb.143.2.926-933.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brubaker R. R. Growth of Pasteurella pseudotuberculosis in simulated intracellular and extracellular environments. J Infect Dis. 1967 Dec;117(5):403–417. doi: 10.1093/infdis/117.5.403. [DOI] [PubMed] [Google Scholar]
- Carter P. B., Zahorchak R. J., Brubaker R. R. Plague virulence antigens from Yersinia enterocolitica. Infect Immun. 1980 May;28(2):638–640. doi: 10.1128/iai.28.2.638-640.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Darveau R. P., Charnetzky W. T., Hurlbert R. E. Outer membrane protein composition of Yersinia pestis at different growth stages and incubation temperatures. J Bacteriol. 1980 Aug;143(2):942–949. doi: 10.1128/jb.143.2.942-949.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveau R. P., Charnetzky W. T., Hurlbert R. F., Hancock R. E. Effects of growth temperature, 47-megadalton plasmid, and calcium deficiency on the outer membrane protein porin and lipopolysaccharide composition of Yersinia pestis EV76. Infect Immun. 1983 Dec;42(3):1092–1101. doi: 10.1128/iai.42.3.1092-1101.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferber D. M., Brubaker R. R. Plasmids in Yersinia pestis. Infect Immun. 1981 Feb;31(2):839–841. doi: 10.1128/iai.31.2.839-841.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemski P., Lazere J. R., Casey T. Plasmid associated with pathogenicity and calcium dependency of Yersinia enterocolitica. Infect Immun. 1980 Feb;27(2):682–685. doi: 10.1128/iai.27.2.682-685.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemski P., Lazere J. R., Casey T., Wohlhieter J. A. Presence of a virulence-associated plasmid in Yersinia pseudotuberculosis. Infect Immun. 1980 Jun;28(3):1044–1047. doi: 10.1128/iai.28.3.1044-1047.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goguen J. D., Yother J., Straley S. C. Genetic analysis of the low calcium response in Yersinia pestis mu d1(Ap lac) insertion mutants. J Bacteriol. 1984 Dec;160(3):842–848. doi: 10.1128/jb.160.3.842-848.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIGUCHI K., KUPFERBERG L. L., SMITH J. L. Studies on the nutrition and physiology of Pasteurella pestis. III. Effects of calcium ions on the growth of virulent and avirulent strains of Pasteurella pestis. J Bacteriol. 1959 Mar;77(3):317–321. doi: 10.1128/jb.77.3.317-321.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIGUCHI K., SMITH J. L. Studies on the nutrition and physiology of Pasteurella pestis. VI. A differential plating medium for the estimation of the mutation rate to avirulence. J Bacteriol. 1961 Apr;81:605–608. doi: 10.1128/jb.81.4.605-608.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HURWITZ J., WEISSBACH A. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. II. Enzymic studies. J Biol Chem. 1959 Apr;234(4):710–712. [PubMed] [Google Scholar]
- KUPFERBERG L. L., HIGUCHI K. Role of calcium ions in the stimulation of growth of virulent strains of Pasteurella pestis. J Bacteriol. 1958 Jul;76(1):120–121. doi: 10.1128/jb.76.1.120-121.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J. H., Walker G. C. Mud(Ap, lac)-generated fusions in studies of gene expression. Methods Enzymol. 1983;100:501–509. doi: 10.1016/0076-6879(83)00075-0. [DOI] [PubMed] [Google Scholar]
- LOVELESS A. The influence of radiomimetic substances on deoxyribonucleic acid synthesis and function studied in Escherichia coli/phage systems. III. Proc R Soc Lond B Biol Sci. 1959 Sep 1;150:497–508. doi: 10.1098/rspb.1959.0038. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Stein S., Hines J. Glucose metabolism in Neisseria gonorrhoeae. J Bacteriol. 1974 Nov;120(2):702–714. doi: 10.1128/jb.120.2.702-714.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Blank H. F., Kingsbury D. T., Falkow S. Genetic analysis of essential plasmid determinants of pathogenicity in Yersinia pestis. J Infect Dis. 1983 Aug;148(2):297–304. doi: 10.1093/infdis/148.2.297. [DOI] [PubMed] [Google Scholar]
- Portnoy D. A., Falkow S. Virulence-associated plasmids from Yersinia enterocolitica and Yersinia pestis. J Bacteriol. 1981 Dec;148(3):877–883. doi: 10.1128/jb.148.3.877-883.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Wolf-Watz H., Bolin I., Beeder A. B., Falkow S. Characterization of common virulence plasmids in Yersinia species and their role in the expression of outer membrane proteins. Infect Immun. 1984 Jan;43(1):108–114. doi: 10.1128/iai.43.1.108-114.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Examination of the protein composition of the cell envelope of Escherichia coli by polyacrylamide gel electrophoresis. J Bacteriol. 1970 Nov;104(2):882–889. doi: 10.1128/jb.104.2.882-889.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Tilghman S. M., Tiemeier D. C., Polsky F., Edgell M. H., Seidman J. G., Leder A., Enquist L. W., Norman B., Leder P. Cloning specific segments of the mammalian genome: bacteriophage lambda containing mouse globin and surrounding gene sequences. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4406–4410. doi: 10.1073/pnas.74.10.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf-Watz H., Portnoy D. A., Bölin I., Falkow S. Transfer of the virulence plasmid of Yersinia pestis to Yersinia pseudotuberculosis. Infect Immun. 1985 Apr;48(1):241–243. doi: 10.1128/iai.48.1.241-243.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahorchak R. J., Brubaker R. R. Effect of exogenous nucleotides on Ca2+ dependence and V antigen synthesis in Yersinia pestis. Infect Immun. 1982 Dec;38(3):953–959. doi: 10.1128/iai.38.3.953-959.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitman D., Ben-Gurion R. Transduction of Pasteurella pestis. Virology. 1972 Feb;47(2):513–516. doi: 10.1016/0042-6822(72)90291-7. [DOI] [PubMed] [Google Scholar]