Abstract
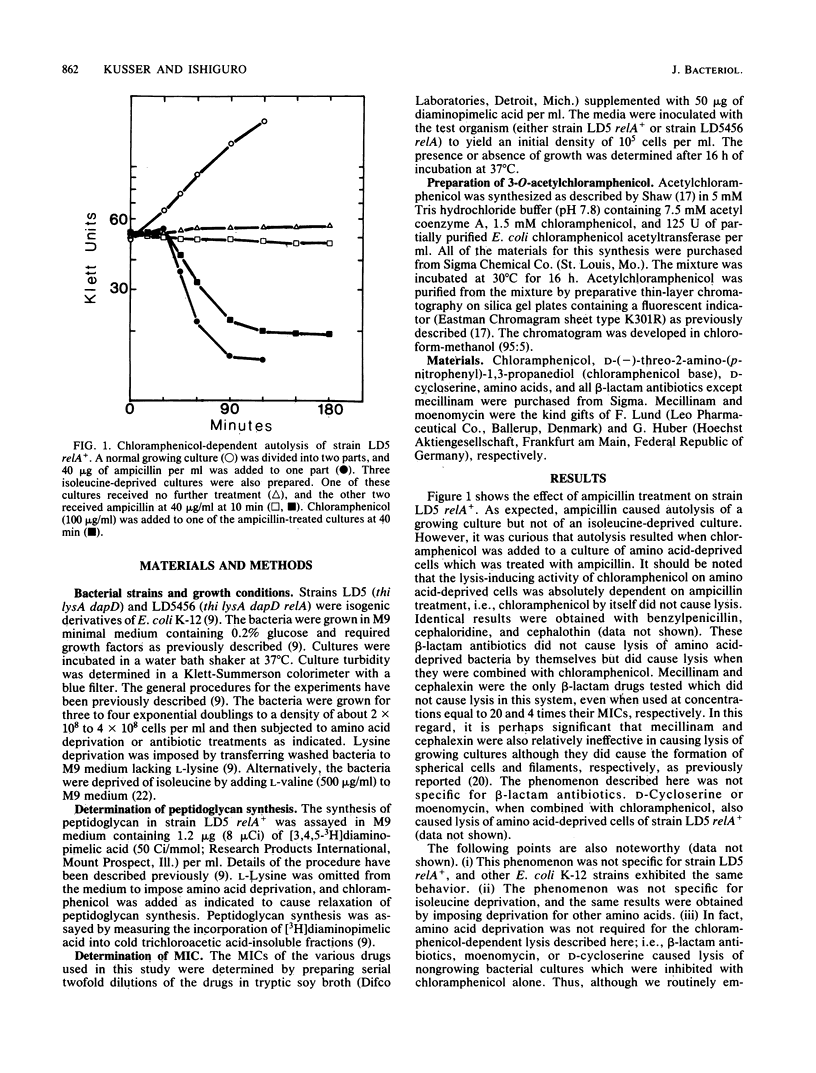
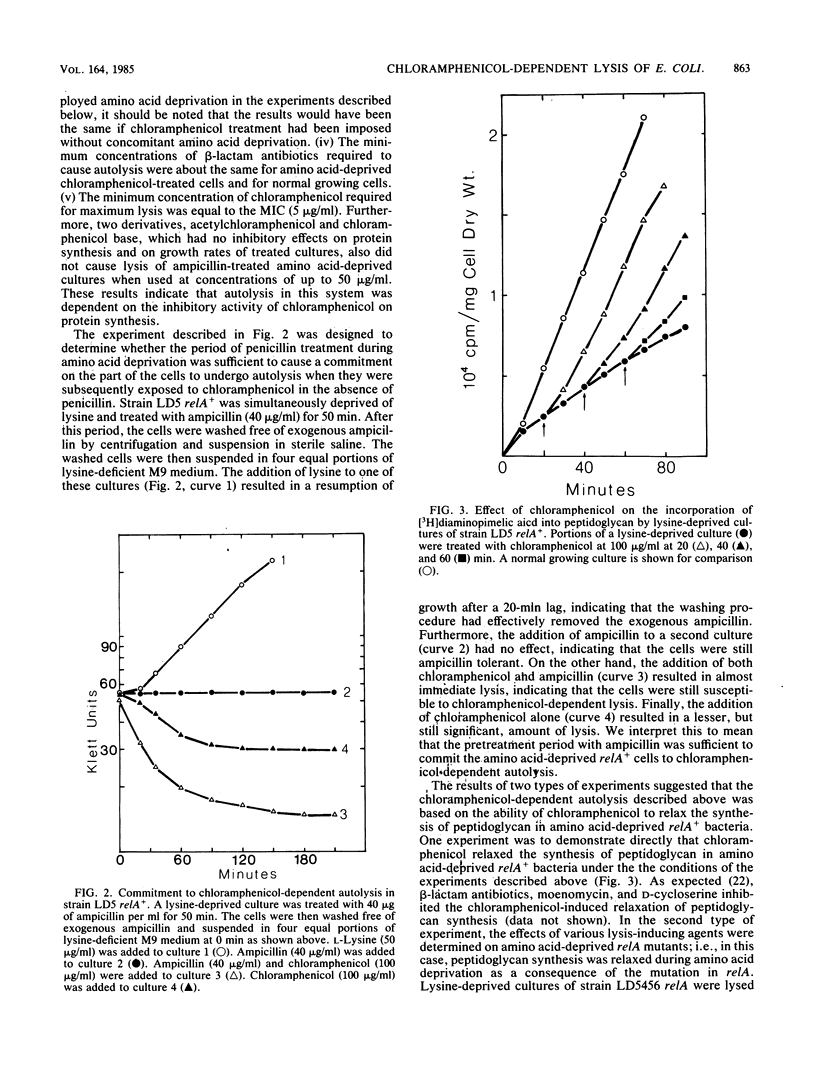
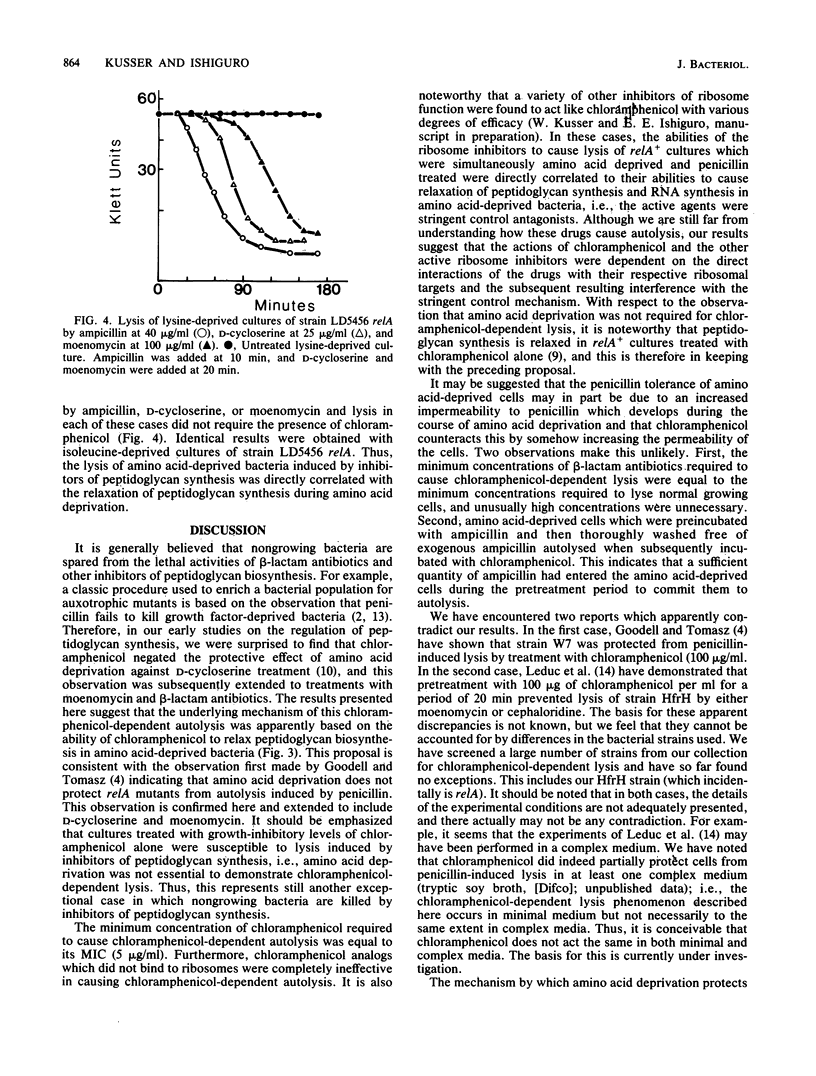
It is generally assumed that inhibitors of peptidoglycan biosynthesis do not kill nongrowing bacteria. An exceptional case is reported here. The addition of chloramphenicol to amino acid-deprived cultures of relA+ strains of Escherichia coli which were treated with beta-lactam antibiotics, D-cycloserine, or moenomycin resulted in lysis. This phenomenon is termed chloramphenicol-dependent lysis. To be effective, chloramphenicol had to be present at its minimum growth-inhibitory concentration (or higher). Analogs of chloramphenicol which did not bind to ribosomes were completely ineffective. Amino acid deprivation was actually not required to demonstrate chloramphenicol-dependent lysis, and cultures treated with growth-inhibitory levels of chloramphenicol alone were lysed when challenged with inhibitors of peptidoglycan synthesis. Peptidoglycan synthesis has been shown previously to be under stringent (relA+) control, and chloramphenicol is known to be an antagonist of stringent control. Thus, it is proposed that the mechanism of chloramphenicol-dependent lysis is based on the ability of chloramphenicol to relax peptidoglycan synthesis in nongrowing relA+ bacteria. This is also consistent with the observation that treatment of amino acid-deprived relA mutants with inhibitors of peptidoglycan synthesis resulted in lysis, i.e., without the mediation of chloramphenicol.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cashel M. Regulation of bacterial ppGpp and pppGpp. Annu Rev Microbiol. 1975;29:301–318. doi: 10.1146/annurev.mi.29.100175.001505. [DOI] [PubMed] [Google Scholar]
- Gallant J. A. Stringent control in E. coli. Annu Rev Genet. 1979;13:393–415. doi: 10.1146/annurev.ge.13.120179.002141. [DOI] [PubMed] [Google Scholar]
- Goodell W., Tomasz A. Alteration of Escherichia coli murein during amino acid starvation. J Bacteriol. 1980 Dec;144(3):1009–1016. doi: 10.1128/jb.144.3.1009-1016.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness R. E., Ishiguro E. E. Temperature-sensitive autolysis-defective mutants of Escherichia coli. J Bacteriol. 1983 Jul;155(1):15–21. doi: 10.1128/jb.155.1.15-21.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro E. E., Mirelman D., Harkness R. E. Regulation of the terminal steps in peptidoglycan biosynthesis in ether-treated cells of Escherichia coli. FEBS Lett. 1980 Nov 3;120(2):175–178. doi: 10.1016/0014-5793(80)80291-2. [DOI] [PubMed] [Google Scholar]
- Ishiguro E. E., Ramey W. D. Involvement of the relA gene product and feedback inhibition in the regulation of DUP-N-acetylmuramyl-peptide synthesis in Escherichia coli. J Bacteriol. 1978 Sep;135(3):766–774. doi: 10.1128/jb.135.3.766-774.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro E. E., Ramey W. D. Stringent control of peptidoglycan biosynthesis in Escherichia coli K-12. J Bacteriol. 1976 Sep;127(3):1119–1126. doi: 10.1128/jb.127.3.1119-1126.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro E. E. Regulation of peptidoglycan biosynthesis in relA+ and relA- strains of Escherichia coli during diauxic growth on glucose and lactose. Can J Microbiol. 1979 Oct;25(10):1206–1208. doi: 10.1139/m79-188. [DOI] [PubMed] [Google Scholar]
- Kitano K., Tomasz A. Escherichia coli mutants tolerant to beta-lactam antibiotics. J Bacteriol. 1979 Dec;140(3):955–963. doi: 10.1128/jb.140.3.955-963.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduc M., Kasra R., van Heijenoort J. Induction and control of the autolytic system of Escherichia coli. J Bacteriol. 1982 Oct;152(1):26–34. doi: 10.1128/jb.152.1.26-34.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramey W. D., Ishiguro E. E. Site of inhibition of peptidoglycan biosynthesis during the stringent response in Escherichia coli. J Bacteriol. 1978 Jul;135(1):71–77. doi: 10.1128/jb.135.1.71-77.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V. The enzymatic acetylation of chloramphenicol by extracts of R factor-resistant Escherichia coli. J Biol Chem. 1967 Feb 25;242(4):687–693. [PubMed] [Google Scholar]
- Shimmin L. C., Vanderwel D., Harkness R. E., Currie B. R., Galloway C. A., Ishiguro E. E. Temperature-sensitive beta-lactam-tolerant mutants of Escherichia coli. J Gen Microbiol. 1984 Jun;130(6):1315–1323. doi: 10.1099/00221287-130-6-1315. [DOI] [PubMed] [Google Scholar]
- Sokawa Y., Nakao E., Kaziro Y. On the nature of the control by RC gene in e. coli: amino acid-dependent control of lipid synthesis. Biochem Biophys Res Commun. 1968 Oct 10;33(1):108–112. doi: 10.1016/0006-291x(68)90263-5. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Penicillin-binding proteins and the future of beta-lactam antibiotics. The Seventh Fleming Lecture. J Gen Microbiol. 1983 May;129(5):1247–1260. doi: 10.1099/00221287-129-5-1247. [DOI] [PubMed] [Google Scholar]
- Tomasz A. The mechanism of the irreversible antimicrobial effects of penicillins: how the beta-lactam antibiotics kill and lyse bacteria. Annu Rev Microbiol. 1979;33:113–137. doi: 10.1146/annurev.mi.33.100179.000553. [DOI] [PubMed] [Google Scholar]
- Vanderwel D., Ishiguro E. E. Properties of cell wall peptidoglycan synthesized by amino acid deprived re1A mutants of Escherichia coli. Can J Microbiol. 1984 Oct;30(10):1239–1246. doi: 10.1139/m84-196. [DOI] [PubMed] [Google Scholar]



