Abstract
Newly synthesized thyroglobulin (Tg), the major secretory glycoprotein of the thyroid gland, folds and homodimerizes in the endoplasmic reticulum (ER) before its export to the site of iodination, where it serves as the precursor for thyroid hormone synthesis. In families with defective Tg export, affected individuals suffer from a thyroidal ER storage disease characterized by a distended thyrocyte ER containing misfolded Tg, along with induced ER molecular chaperones. Inherited as an autosomal recessive trait, deficient Tg causes congenital hypothyroidism in newborns that, if untreated, results in goiter along with serious cognitive and growth defects. Recently, a similar phenotype has been observed in inbred cog/cog mice, although the precise molecular defect has remained undefined. Here, we have isolated and cloned a full-length 8.5-kb Tg cDNA from cog/cog mice and unaffected isogenic AKR/J mice. Comparison of the complete sequences reveals that cog/cog mice express a Leu-2263 → Pro missense mutation in the acetylcholinesterase-homology domain of Tg. Heterologous expression studies in COS cells indicate that cog Tg exhibits a severe defect in exit from the ER. Site-directed mutagenesis of cog Tg to convert the single amino acid back to Leu-2263 restores normal Tg secretion. We conclude that the cog mutation in Tg is responsible for this ER storage disease that causes thyroid dyshormonogenesis.
For trafficking through the eukaryotic secretory pathway, exportable proteins must achieve a certain level of conformational maturity to be competent for intracellular transport as well as for their ultimate biological function. An increasing number of genetically transmitted metabolic diseases involving intracellular retention of exportable proteins have been recognized, caused by mutations that lead to tertiary and quaternary structural defects (1). In a family of disorders of protein trafficking known as endoplasmic reticulum storage diseases (ERSDs; ref. 2), mutations as small as a single amino acid change can lead to failure of protein export from the ER (an essential step in overall protein export), such as in cystic fibrosis [mutant CFTR (3)], juvenile pulmonary emphysema [mutant α-l-antitrypsin (4)], osteogenesis imperfecta [mutant type I procollagen (5)], juvenile diabetes insipidus [mutant vasopressin precursor (6)], and hypercholesterolemia [mutant low density lipoprotein receptor (7)]. Because export of the mutant protein from the ER is prevented, it never reaches the destination at which its physiologic function normally takes place.
Mutations in thyroglobulin (Tg), the major thyroid secretory glycoprotein, can also cause inherited disease (2, 8, 9). Normally, Tg is exported through the secretory pathway to the lumen of thyroid follicles to undergo iodination, which leads to thyroid hormone synthesis. Congenital hypothyroidism due to deficient Tg is inherited as an autosomal recessive trait in ≈1:40,000 human newborns (10). In several human kindreds that have been studied with this disorder, defective trafficking of Tg from the ER to Golgi has been suggested to be responsible for the congenital thyroid hormone deficiency (11). In at least one of these kindreds, the underlying molecular defect has been proved to be a mutation resulting in an altered Tg coding sequence (12).
A similar form of severe congenital hypothyroidism with colloid-deficient goiter along with abnormal growth and central nervous system development is observed in cog/cog mice (13). Indeed, electron microscopic studies of the thyroid glands of cog/cog mice indicate an abnormally distended ER (14)—a phenotype comparable to that observed from thyroid biopsies of children suffering from congenital goiter with defective Tg (11, 15–17). In the cog/cog mouse, Tg mRNA is abundant and normal in size, but purified Tg protein exhibits abnormal biophysical properties including enhanced susceptibility to proteolysis (18–20). We have recently established that abnormal folding, dimerization, and export of Tg, in association with markedly elevated levels of five ER molecular chaperones, characterizes the thyroid defect, indicating an ERSD (21). Moreover, in thyroid tissue from homozygous cog/cog mice, Tg exhibits temperature-sensitive export from the ER, suggesting that the cog defect might be caused by a point mutation in the Tg coding sequence (21).
The cog trait originally appeared as a spontaneous autosomal recessive phenotype in the inbred AKR/J strain of mice (22). The cog gene has been mapped near the Tg gene locus at the central region of mouse chromosome 15 (23), but the precise genetic defect has remained elusive. Here, we have prepared thyroid cDNA libraries from cog/cog and isogenic AKR/J mice, which, by cDNA sequencing, has allowed us to deduce the complete Tg primary structure in both cases. These data demonstrate that the hormonogenic domains of cog Tg are identical to those of the normal Tg. However, we have found a single nucleotide substitution in cog Tg, changing Leu-2263 to Pro-2263 in a region strictly conserved in the Tgs from all known species, which is evolutionarily derived from acetylcholinesterase (AChE) and is known to be essential for the structural stability of the AChE molecule. Heterologous expression studies confirm that cog Tg exhibits deficient intracellular transport, whereas PCR-mediated reversion of Pro-2263 to Leu-2263 corrects the defect. Thus, the Leu-2263 → Pro mutation in cog Tg is responsible for the ERSD that causes thyroid dyshormonogenesis.
MATERIALS AND METHODS
Animals.
Homozygous cog/cog and AKR/J mice were purchased from The Jackson Laboratories. At 6 months of age, thyroid goiters were removed from four mutant mice for generation of a thyroid cDNA library. For the control cDNA library, freshly isolated thyroids from 12 AKR/J mice were employed.
Isolation and Sequencing of Tg cDNAs.
Using the Gubler–Hoffman technique (24), thyroid cDNA libraries from cog/cog and AKR/J mice were generated. Bidirectional DNA strands were synthesized in a single tube without intervening extractions. Because Tg is encoded by a long message, the initial reaction using oligo(dT) (containing a terminal XhoI site) employed Superscript II reverse transcriptase (Moloney murine leukemia virus-reverse transcriptase devoid of RNase H from GIBCO/BRL). The first cDNA strand was protected by methylation. Fragments of mRNA that had been nicked by RNase H served as primers for DNA polymerase I in making the second cDNA strand. Following ligation of an EcoRI linker and digestion with XhoI, double-stranded cDNAs were then cloned into predigested λ arms of the ZAP express vector (Stratagene). The bacteriophage libraries contained a primary titer of >1 million plaque-forming units/ml.
Seven clones from the cog/cog library, presumably representing the full-length (≥8.2 kb) Tg cDNA, were initially selected by probing with a cDNA fragment containing 1.7 kb of the extreme 5′ coding sequence of rat Tg (25). Five positive clones gave identical restriction patterns when digested with EcoRI and XhoI. By using the enzymatic dideoxy method (26), the terminal 100 bp at the 5′ and 3′ ends of the coding region were found to be identical for all five clones. Three of these full-length clones that contained the most 5′-untranslated sequence were then subcloned into pBS+ (Stratagene). One such clone was then selected for sequencing (see Fig. 1), beginning with standard T3 and T7 oligonucleotide primers. The Tg cDNA was also digested with BamHI and PstI and appropriate fragments ligated into pBS+, so that additional internal sequence could be obtained from reactions by using these same standard primers. The remaining internal cDNA sequence was obtained from reactions by using primers designed on the basis of cog Tg sequences that were available from earlier sequencing runs. Using this approach, the cog Tg cDNA was completely sequenced in both directions, which helped to eliminate sequencing errors. Then, by using specific cog Tg cDNA fragments as probes, several large and overlapping fragments from the AKR/J library were isolated and sequenced (no single full-length Tg cDNA clone was isolated from this library). From this approach, the entire Tg coding sequence from the control mice was obtained. All sequencing was performed in the DNA sequencing facility at Beth Israel Hospital (Boston).
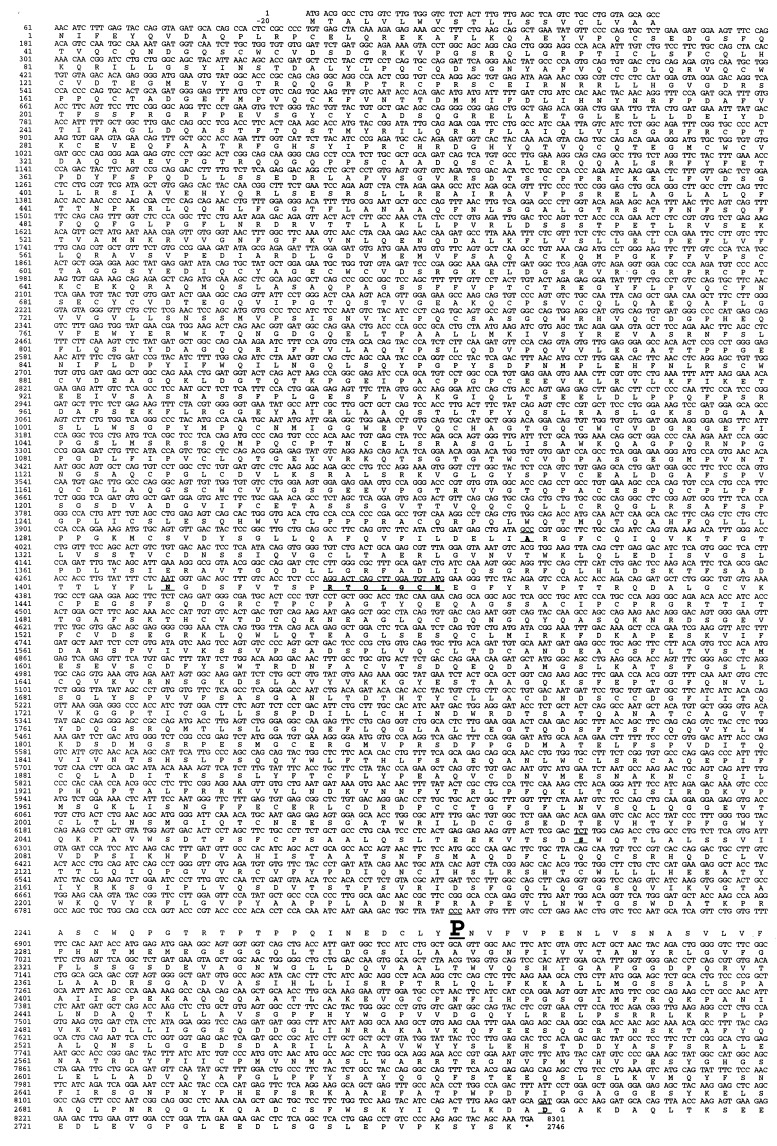
Figure 1.
Tg cDNA sequence of cog/cog mice with congenital hypothyroid goiter differs by one nucleotide from that of euthyroid AKR/J mice. The complete cog Tg coding sequence and the primary structure of the deduced cog Tg protein are shown. The preparation of thyroid cDNA libraries from the two isogenic strains of mice, library screening, and DNA sequencing of selected clones, is described in Materials and Methods. The deduced cog Tg protein sequence differs in six locations from that recently reported in GenBank (accession no. U76389) for normal outbred mice (the differing codons are shown as underlined areas). Of these differences, only the nucleotide substitution (CTC to CCC) at position 6848, resulting in a Leu-2263 → Pro mutation which resides in the acetylcholinesterase-homology domain of Tg, differs between the Tg sequences of cog/cog and AKR/J mice.
Transient Expression of Mutant and Normal Tg.
COS-7 cells were grown in DMEM containing 10% FBS. Using lipofectamine (GIBCO/BRL), subconfluent cells were then tranfected either with the full-length cog Tg or normal Tg cDNA, both subcloned into the pBK expression vector (Stratagene); a nonmutant Tg cDNA in the pCB6 expression vector (27) was used in Fig. 2c. These vectors drive Tg expression from the immediate early cytomegalovirus promoter. At 48 h after transfection, the medium was changed to include 10 mM Na butyrate and the cells further incubated for 1 day. The bathing medium was then collected and precipitated with trichloroacetic acid, while the cells were lysed in 50 mM Tris (pH 7.4) plus boiling 2% SDS and 25 mM DTT. Both medium and cell lysate samples were then diluted into gel sample buffer, resolved by 5% SDS/PAGE, and electrophoretically transferred to nitrocellulose. Immunoblotting employed rabbit polyclonal anti-Tg sera, with a goat anti-rabbit secondary antibody coupled to peroxidase. Blots were developed by using enhanced chemiluminescence with luminol. For immunoprecipitation analysis, transfected COS-7 cells were pulse-labeled with [35S]methionine/cysteine and chased for various times prior to lysis in a nondenaturing buffer containing a standard mixture of protease inhibitors (28). Equal aliquots of cell lysates or chase medium were precleared with Zysorbin prior to immunoprecipitation with polyclonal anti-Tg, followed by reducing SDS/PAGE. Endoglycosidase H digests were performed as described (21). Tg bands were detected by phosphorimaging (Molecular Dynamics).
Figure 2.
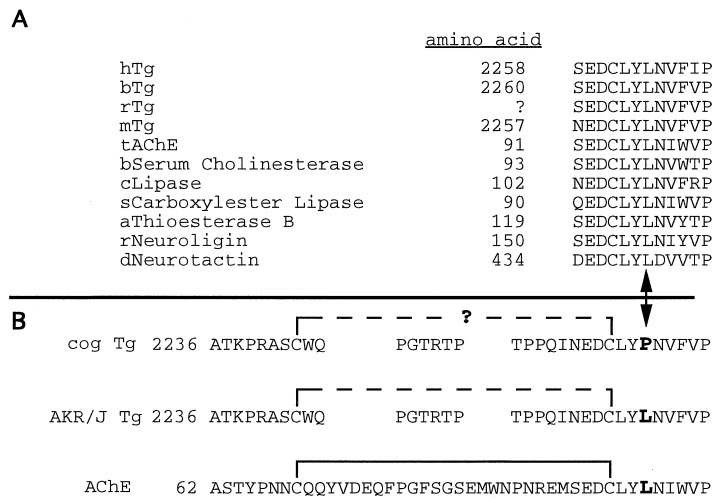
The cog mutation is located within a conserved structural domain. (A) The amino acids surrounding Leu-2263 (denoted with an arrowhead, at bottom) are highly conserved in Tg from all four species that have thus far been examined, as well as in AChE and other members of the “α/β hydrolase fold” family (a partial list is shown; h, human; b, bovine; r, rat; m, mouse; t, torpedo; c, candida; s, salmon; a, avian; d, Drosophila). The amino acid numbering for rat Tg is not established because the cDNA sequence has not yet been determined in its entirety. (B) Given the close proximity of Leu-2263 to Cys-2260, which forms an intrachain disulfide bond (solid bracket) that provides important structural stability to AChE (see text) and is likely to play a similar role in Tg (dashed bracket), we hypothesize that the cog mutation (L2263P) may destabilize this region, potentially by preventing proper formation of this disulfide bridge (signified by question mark).
RESULTS AND DISCUSSION
By detecting for both the 5′ and 3′ ends of the Tg cDNA, five independent clones that contained full-length inserts (≥8.2 kb) were isolated from a thyroid cDNA library prepared from cog/cog mice (see Materials and Methods). Complete, bidirectional DNA sequence obtained for one clone (referred to as cog Tg) included an 8301-nt insert containing an ORF region encoding an intact pre-prohormone that contained a 20-residue signal peptide and a 2746-residue mature protein, consistent with a full-length Tg translation product (Fig. 1). When compared with the Tg sequence recently reported in GenBank for normal outbred mice, the deduced cog Tg translation product differed in six locations (Fig. 1, underlines), reflecting heterogeneity among murine Tgs, similar to that reported to occur between normal humans (29). To determine which if any of these six sequence differences might be responsible for the cog phenotype, we also prepared a thyroid cDNA library and obtained the complete Tg cDNA sequence from AKR/J mice, the original parental inbred strain that does not exhibit any thyroid abnormality. Because the cog/cog and AKR/J stains are isogenic, if the cog defect is found in the Tg coding sequence, then only a single region of sequence difference is to be expected, whereas if the cog gene is not the Tg gene, the two Tg sequences should be identical.
When the two Tg cDNAs were compared, the sole observed difference was a CTC → CCC substitution at position 6848 of the ORF, creating the mutation Leu-2263 → Pro in the mature cog Tg protein (Fig. 1, bold type). This was confirmed by selective sequencing of two independently isolated, full-length cog Tg cDNA clones. The proline substitution is not one of the sequence variations reported for normal outbred mice (Fig. 1). Importantly, the wild-type leucine residue in this position is conserved in all species for which suitable Tg sequence has been reported (Fig. 2a).
The C-terminal 544 amino acids of Tg exhibit significant homology to the entirety of acetylcholinesterase (AChE; refs. 30–32), which shares with Tg the features of dimer formation (33, 34) and transport through the secretory pathway (34). The highest degree of homology is contained in a region conserved between virtually all members of the large “α/β hydrolase fold” family, including hydrolytic enzymes and cell adhesion molecules that are thought to have diverged from a common ancestral gene (35). This conserved region in the crystal structure of AChE (Fig. 2a), and presumably in other family members, is contained within a peptide loop connecting antiparallel β-strands (33) that are thought to play a critical structural role.
An expanded view (Fig. 2b) demonstrates that an intrachain disulfide bond, which is adversely affected by changes in the neighboring amino acids (36), is in close proximity to the conserved peptide loop in AChE. Notably, a Asp → Asn substitution in this conserved sequence has been shown to result in deficient protein export (36), a consequence of structural instability that probably represents either failure to form the intrachain disulfide bond (Fig. 2b) or the proper antiparallel alignment of β-strands. Based on such observations, we hypothesize that a structurally critical disulfide bond also exists in this region of Tg, and that the neighboring cog mutation may cause similar structural instability leading to deficient Tg export. Given that homodimerization of Tg occurs the ER (37), the structural instability provided by mutations in this region can account for the established deficiency of cog Tg in homodimerization and intracellular transport (21). This in turn can explain the abnormally distended thyrocyte ER with a vast induction of molecular chaperones (21).
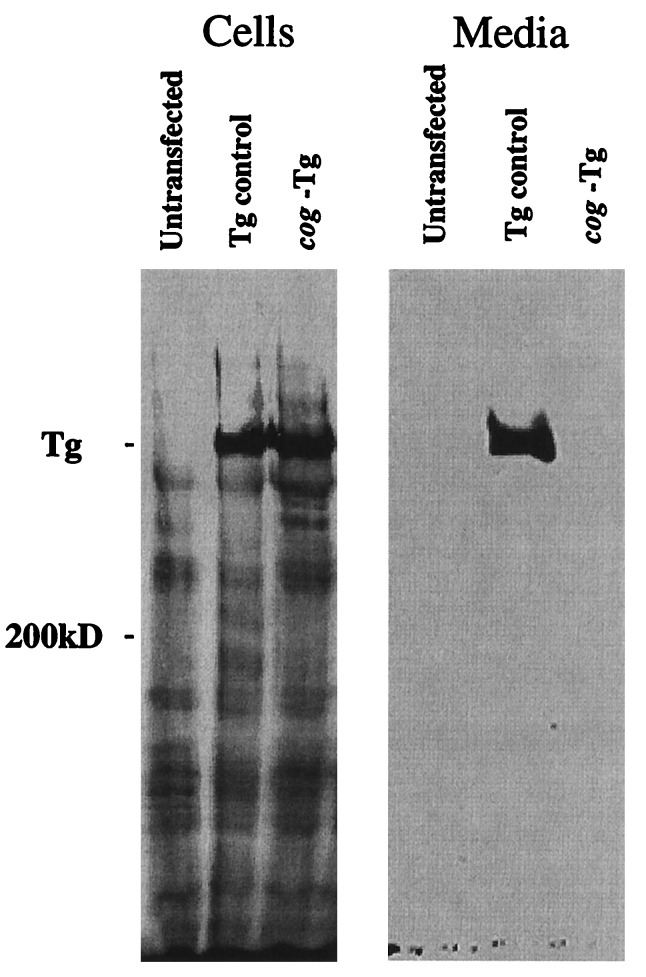
By using the cog Tg cDNA, we transiently expressed the protein in COS cells. As shown by immunoblotting, untransfected COS cells contained only background bands in cell lysates (Fig. 3 Left) and no bands in the medium (Fig. 3 Right). In addition, COS cells transfected with a control cDNA encoding nonmutant Tg exhibited a specific Tg band intracellularly and secreted into the medium. By contrast, the expressed cog Tg protein was detectable only intracellularly (Fig. 3). These data confirm that the cloned cog cDNA encodes a Tg protein defective for secretion.
Figure 3.
Defective intracellular trafficking of cog Tg in COS cells. COS-7 cells were either untransfected or transiently tranfected with Tg cDNAs as described in Materials and Methods. By Western blot analysis, unlike the control Tg, a protein encoded by the cog Tg cDNA could be found intracellularly but was secreted into the overnight collection medium at levels below the limits of detection.
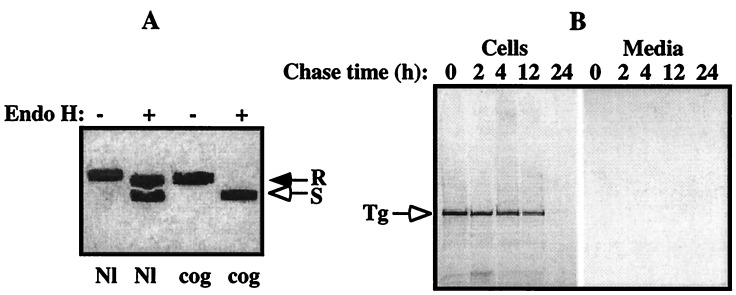
To determine if the recombinantly expressed cog Tg ever reached the Golgi complex, we examined its sensitivity to digestion with endoglycosidase H (21). As shown in Fig. 4a, cog Tg remained completely sensitive to digestion, indicating that the mutant protein was retained in the ER. Moreover, we found that the fate of transiently expressed cog Tg involves intracellular degradation as a function of time (Fig. 4b). Although the kinetics of degradation appeared relatively slow, it is consistent with the recently described ER-associated degradation process (38) that occurs in many ERSDs (2). Studies are currently ongoing to characterize this process for Tg in greater detail.
Figure 4.
Cog Tg is retained in the ER and degraded intracellularly. COS-7 cells, transfected with nonmutant or cog Tg cDNAs as in Fig. 3, were pulse-labeled with [35S]methionine/cysteine, chased for various times, and the medium collected and cells lysed as described. (A) Equal aliquots of cell lysates and chase medium were combined and immunoprecipitated with polyclonal antiserum against mouse Tg, prior to digestion with endo H (+) or mock digestion (−). As shown at 6 h of chase, ≥60% of normal Tg had passed the medial Golgi as measured by acquisition of resistance to endo H digestion (R) with secretion into the medium (not shown), whereas cog Tg remained entirely sensitive (S) to endo H digestion (open arrow), indicating that the mutant Tg protein never reached the Golgi for complex carbohydrate addition. (B) Equal aliquots of lysates of cells transfected with the cog Tg cDNA, and the corresponding chase medium samples, were immunoprecipitated with antisera against Tg and analyzed by SDS/PAGE and fluorography. Although ≈40% of cog Tg is slowly degraded over the first 12 h, during the second 12-h chase, the remaining newly synthesized cog Tg was more rapidly degraded with an undetectable fraction appearing in the secreted media, consistent with biphasic degradation kinetics (39).
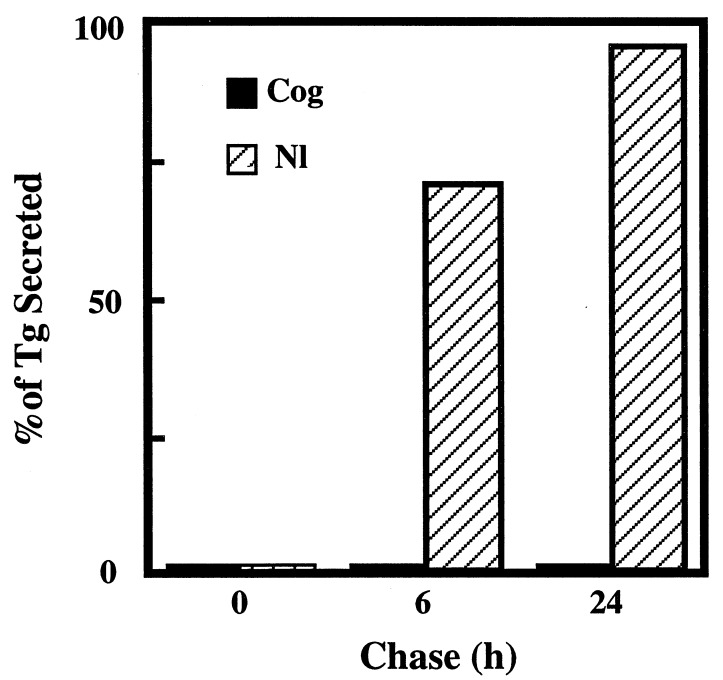
Finally, to prove that the transport defect in cog Tg can be specifically attributed to Pro-2263, we used PCR-based mutagenesis to specifically convert the cog Tg cDNA back to wild-type only at residue Leu-2263. As shown in Fig. 5, correction of this single residue restored normal Tg secretion, proving that the conserved leucine in this region is essential for the structural stability (21) and intracellular transport (this report) of the Tg protein.
Figure 5.
Pro-2263 → Leu-corrected Tg is secreted like the wild-type protein. Using PCR-mutagenesis, the cog Tg cDNA was converted back to wild-type at residue Leu-2263. COS-7 cells, transfected with either the mutant or Pro-2263 → Leu-corrected Tg cDNA, were examined by immunoprecipitation, and SDS/PAGE as in Fig. 4. Radiolabeled Tg bands were quantitated by phosphorimaging.
In conclusion, we have identified a point mutation contained within the AChE-homology domain of the Tg coding sequence as the molecular basis for congenital hypothyroid goiter in cog/cog mice, a prototypic ERSD. Further, though formally possible, it is unlikely that the cog/cog mouse has a second genetic lesion. Future work will be aimed at precisely defining how the AChE-like domain may regulate the stability and assembly of Tg homodimers. Finally, although the molecular lesion is known in relatively few human cases, the fact that a similar thyroid ERSD is observed in numerous patients suffering from congenital hypothyroid goiter suggests that subtle defects in the Tg coding sequence in these kindreds may be expected.
Acknowledgments
This work was supported by National Institutes of Health Grants DK-40344 to P.A. and DK-02113 and DK-52076 to P.S.K., as well as support from Knoll Pharmaceuticals.
ABBREVIATIONS
- Tg
thyroglobulin
- ERSD
endoplasmic reticulum storage disease
- AChE
acetylcholinesterase
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF076186).
References
- 1.Hammond C, Helenius A. Curr Opin Cell Biol. 1995;7:523–529. doi: 10.1016/0955-0674(95)80009-3. [DOI] [PubMed] [Google Scholar]
- 2.Kim P S, Arvan P. Endocr Rev. 1998;19:173–202. doi: 10.1210/edrv.19.2.0327. [DOI] [PubMed] [Google Scholar]
- 3.Ward C L, Omura S, Kopito R R. Cell. 1995;83:121–127. doi: 10.1016/0092-8674(95)90240-6. [DOI] [PubMed] [Google Scholar]
- 4.Sifers R N, Finegold M J, Woo S L. Semin Liver Dis. 1992;12:301–310. doi: 10.1055/s-2008-1040399. [DOI] [PubMed] [Google Scholar]
- 5.Prockop D J, Kivirikko K I. Annu Rev Biochem. 1995;64:403–434. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- 6.Ito M, Jameson J L, Ito M. J Clin Invest. 1997;99:1897–1905. doi: 10.1172/JCI119357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pathak R K, Merkle R K, Cummings R D, Goldstein J L, Brown M S, Anderson R G. J Cell Biol. 1988;106:1831–1841. doi: 10.1083/jcb.106.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medeiros-Neto G, Stanbury J B. Inherited Disorders of the Thyroid System. Boca Raton, FL: CRC; 1994. pp. 107–138. , 221. [Google Scholar]
- 9.Ricketts M H, Simons M J, Parma J, Mercken L, Dong Q, Vassart G. Proc Natl Acad Sci USA. 1987;84:3181–3184. doi: 10.1073/pnas.84.10.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ieiri T, Cochaux P, Targovnik H M, Suzuki M, Shimoda S, Perret J, Vassart G. J Clin Invest. 1991;88:1901–1905. doi: 10.1172/JCI115513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medeiros-Neto G, Kim P S, Yoo S E, Vono J, Targovnik H, Camargo R, Hossain S A, Arvan P. J Clin Invest. 1996;98:2838–2844. doi: 10.1172/JCI119112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Targovnick H M, Vono J, Billerbeck E C, Cerrone G E, Varela V, Mendive F, Wajchenberg B L, Medeiros-Neto G. JCEM. 1995;80:3356–3360. doi: 10.1210/jcem.80.11.7593451. [DOI] [PubMed] [Google Scholar]
- 13.Sugisaki T, Beamer W G, Noguchi T. Neurochem Res. 1992;17:1037–1040. doi: 10.1007/BF00966833. [DOI] [PubMed] [Google Scholar]
- 14.Mayerhofer A, Amador A G, Beamer W G, Bartke A. J Hered. 1988;79:200–203. doi: 10.1093/oxfordjournals.jhered.a110492. [DOI] [PubMed] [Google Scholar]
- 15.Michel-Bechet M, Cotte G, Codaccioni J-L, Athouel-Haon A-M. Acta Anat. 1969;73:389–409. [PubMed] [Google Scholar]
- 16.Lissitzky S, Torresani J, Burrow G N, Bouchilloux S, Chabaud O. Clin Endocrinol. 1975;4:363–392. doi: 10.1111/j.1365-2265.1975.tb01545.x. [DOI] [PubMed] [Google Scholar]
- 17.Ohyama Y, Hosoya T, Kameya T, Suzuki N, Nakamura S, Kazahari K, Shibayama K, Yokota Y, Matsura N. Clin Endocrinol. 1994;41:129–135. doi: 10.1111/j.1365-2265.1994.tb03794.x. [DOI] [PubMed] [Google Scholar]
- 18.Basche M, Beamer W G, Schneider A B. Endocrinology. 1989;124:1822–1829. doi: 10.1210/endo-124-4-1822. [DOI] [PubMed] [Google Scholar]
- 19.Adkison L R, Taylor S, Beamer W G. J Endocrinol. 1990;126:51–58. doi: 10.1677/joe.0.1260051. [DOI] [PubMed] [Google Scholar]
- 20.Fogelfeld L, Harel G, Beamer W G, Schneider A B. Thyroid. 1992;2:329–335. doi: 10.1089/thy.1992.2.329. [DOI] [PubMed] [Google Scholar]
- 21.Kim P S, Kwon O-Y, Arvan P. J Cell Biol. 1996;133:517–527. doi: 10.1083/jcb.133.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beamer W G, Maltais L J, DeBaets M H, Eicher E M. Endocrinology. 1987;120:838–840. doi: 10.1210/endo-120-2-838. [DOI] [PubMed] [Google Scholar]
- 23.Taylor B A, Rowe L. Proc Natl Acad Sci USA. 1987;84:1986–1990. doi: 10.1073/pnas.84.7.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gubler U, Hoffman B J. Gene. 1983;25:263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- 25.Graves P N, Davies T F. Mol Endocrinol. 1990;4:155–161. doi: 10.1210/mend-4-1-155. [DOI] [PubMed] [Google Scholar]
- 26.Sanger F, Air G M, Barrell B G, Brown N L, Coulson A R, Fiddes C A, Hutchison C A, Slocombe P M, Smith M. Nature (London) 1977;265:687–695. doi: 10.1038/265687a0. [DOI] [PubMed] [Google Scholar]
- 27.Muresan Z, Arvan P. J Biol Chem. 1997;272:26095–26102. doi: 10.1074/jbc.272.42.26095. [DOI] [PubMed] [Google Scholar]
- 28.Kim P, Bole D, Arvan P. J Cell Biol. 1992;118:541–549. doi: 10.1083/jcb.118.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van de Graaf S A, Pauws E, de Vijlder J J, Ris-Stalpers C R. Eur J Endocrinol. 1997;136:508–515. doi: 10.1530/eje.0.1360508. [DOI] [PubMed] [Google Scholar]
- 30.Schumacher M, Camp S, Maulet Y, Newton M, MacPhee-Quigley K, Taylor S S, Friedmann T, Taylor P. Nature (London) 1986;319:407–409. doi: 10.1038/319407a0. [DOI] [PubMed] [Google Scholar]
- 31.Swillens S, Ludgate M, Mercken L, Dumont J E, Vassart G. Biochem Biophys Res Commun. 1986;137:142–148. doi: 10.1016/0006-291x(86)91187-3. [DOI] [PubMed] [Google Scholar]
- 32.Mori N, Itoh N, Salvaterra P M. Proc Natl Acad Sci USA. 1987;84:2813–2817. doi: 10.1073/pnas.84.9.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sussman J L, Harel M, Frolow F, Oefner C, Goldman A, Toker L, Silman I. Science. 1991;253:872–879. doi: 10.1126/science.1678899. [DOI] [PubMed] [Google Scholar]
- 34.Bourne Y, Taylor P, Marchot P. Cell. 1995;83:503–512. doi: 10.1016/0092-8674(95)90128-0. [DOI] [PubMed] [Google Scholar]
- 35.Cousin X, Hotelier T, Giles K, Toutant J P, Chatonnet A. Nucleic Acids Res. 1998;26:228–230. doi: 10.1093/nar/26.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shafferman A, Kronman C, Flashner Y, Leitner M, Grosfeld H, Ordentlich A, Gozes Y, Cohen S, Ariel N, Barak D, Harel M, Silman I, Sussman J L, Velan B. J Biol Chem. 1992;267:17640–17648. [PubMed] [Google Scholar]
- 37.Kim P S, Arvan P. J Biol Chem. 1991;266:12412–12418. [PubMed] [Google Scholar]
- 38.Brodsky J L, McCraken A A. Trends Cell Biol. 1997;7:151–156. doi: 10.1016/S0962-8924(97)01020-9. [DOI] [PubMed] [Google Scholar]
- 39.Tsao Y S, Ivessa N E, Adesnik M, Sabatini D D, Kreibich G. J Cell Biol. 1992;116:57–67. doi: 10.1083/jcb.116.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]