Abstract
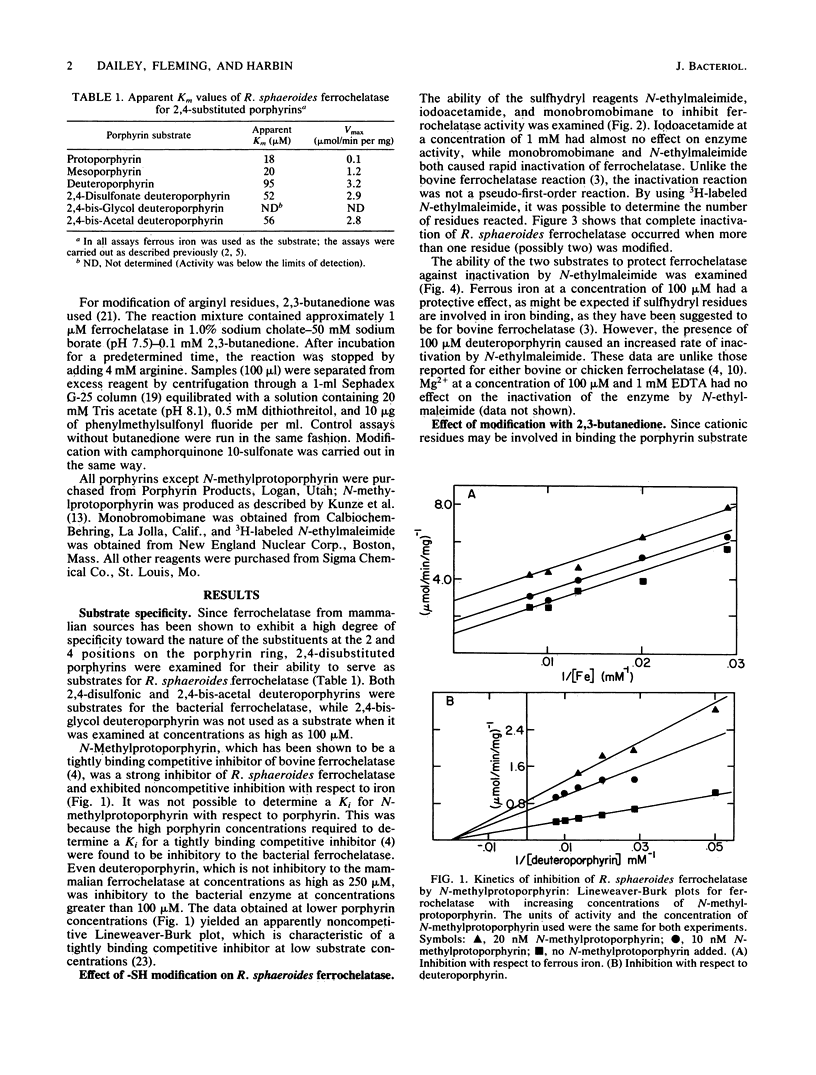
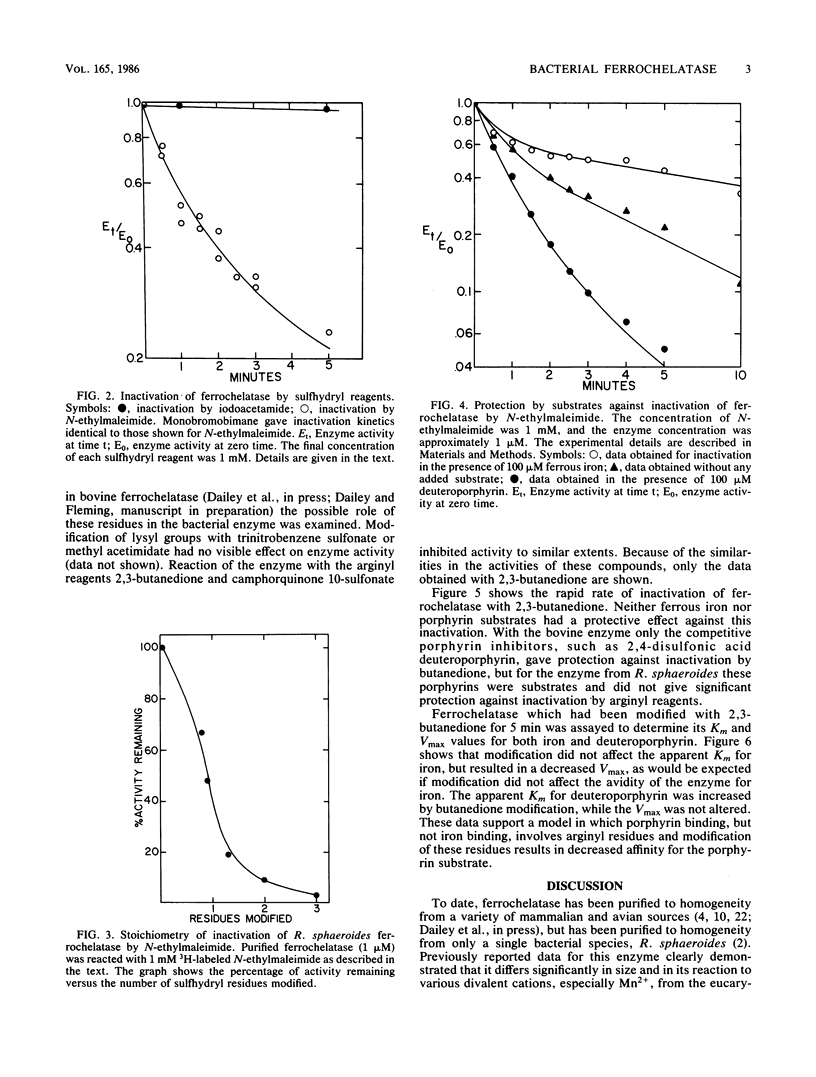
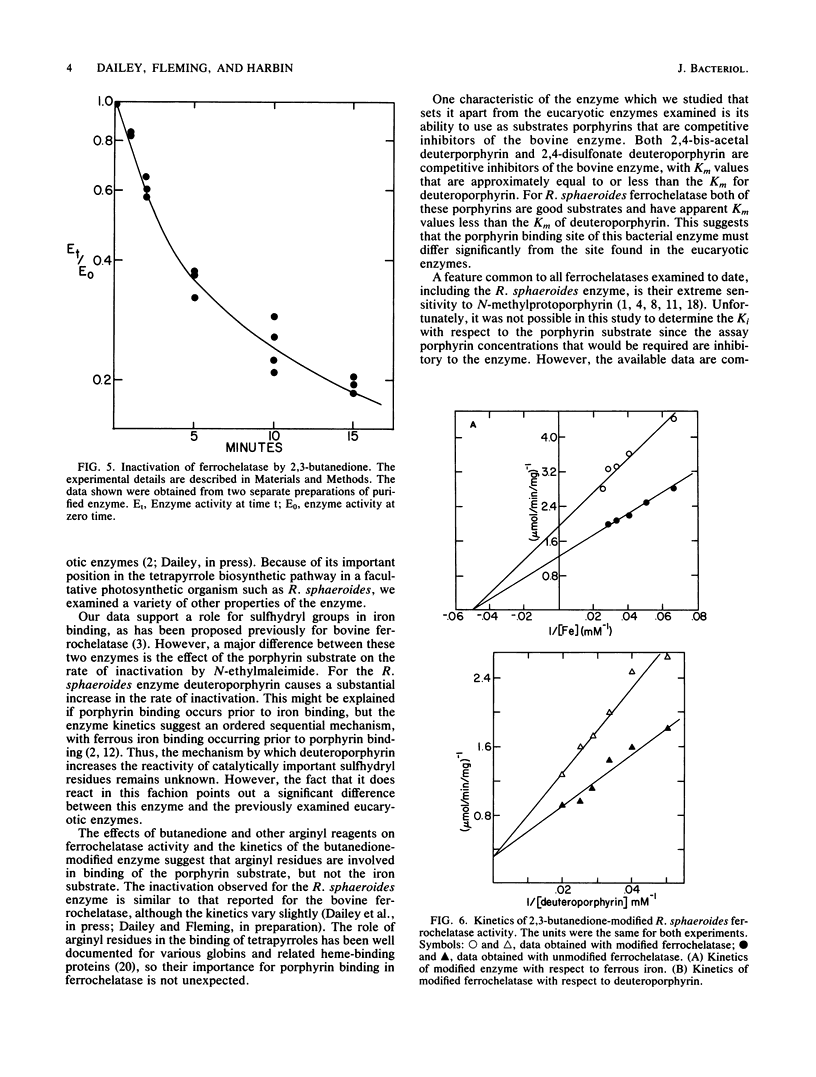
Purified ferrochelatase (protoheme ferrolyase; EC 4.99.1.1) from the bacterium Rhodopseudomonas sphaeroides was examined to determine the roles of cationic and sulfhydryl residues in substrate binding. Reaction of the enzyme sulfhydryl residues with N-ethylmaleimide or monobromobimane resulted in a rapid loss of enzyme activity. Ferrous iron, but not porphyrin substrate, had a protective effect against inactivation by these two reagents. Quantitation with 3H-labeled N-ethylmaleimide revealed that inactivation required one to two sulfhydryl groups to be modified. Modification of arginyl residues with either 2,3-butanedione or camphorquinone 10-sulfonate resulted in a loss of ferrochelatase activity. A kinetic analysis of the modified enzyme showed that the Km for ferrous iron was not altered but that the Km for the porphyrin substrate was increased. These data suggested that arginyl residues may be involved in porphyrin binding, possibly via charge pair interactions between the arginyl residue and the anionic porphyrin propionate side chain. Modification of lysyl residues had no effect on enzyme activity. We also examined the ability of bacterial ferrochelatase to use various 2,4-disubstituted porphyrins as substrates. We found that 2,4-bis-acetal- and 2,4-disulfonate deuteroporphyrins were effective substrates for the purified bacterial enzyme and that N-methylprotoporphyrin was an effective inhibitor of the enzyme. Our data for the ferrochelatase of R. sphaeroides are compared with previously published data for the eucaryotic enzyme.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown S. B., Holroyd J. A., Vernon D. I., Troxler R. F., Smith K. M. The effect of N-methylprotoporphyrin IX on the synthesis of photosynthetic pigments in Cyanidium caldarium. Further evidence for the role of haem in the biosynthesis of plant billins. Biochem J. 1982 Nov 15;208(2):487–491. doi: 10.1042/bj2080487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey H. A. Effect of sulfhydryl group modification on the activity of bovine ferrochelatase. J Biol Chem. 1984 Mar 10;259(5):2711–2715. [PubMed] [Google Scholar]
- Dailey H. A., Fleming J. E. Bovine ferrochelatase. Kinetic analysis of inhibition by N-methylprotoporphyrin, manganese, and heme. J Biol Chem. 1983 Oct 10;258(19):11453–11459. [PubMed] [Google Scholar]
- Dailey H. A., Jr, Lascelles J. Reduction of iron and synthesis of protoheme by Spirillum itersonii and other organisms. J Bacteriol. 1977 Feb;129(2):815–820. doi: 10.1128/jb.129.2.815-820.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey H. A., Jr Purification and characterization of the membrane-bound ferrochelatase from Spirillum itersonii. J Bacteriol. 1977 Oct;132(1):302–307. doi: 10.1128/jb.132.1.302-307.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey H. A. Purification and characterization of membrane-bound ferrochelatase from Rhodopseudomonas sphaeroides. J Biol Chem. 1982 Dec 25;257(24):14714–14718. [PubMed] [Google Scholar]
- Dailey H. A., Smith A. Differential interaction of porphyrins used in photoradiation therapy with ferrochelatase. Biochem J. 1984 Oct 15;223(2):441–445. doi: 10.1042/bj2230441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F., Gibbs A. H., Smith A. G. Inhibition of protohaem ferro-lyase by N-substituted porphyrins. Structural requirements for the inhibitory effect. Biochem J. 1980 Sep 1;189(3):645–648. doi: 10.1042/bj1890645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granick S., Beale S. I. Hemes, chlorophylls, and related compounds: biosynthesis and metabolic regulation. Adv Enzymol Relat Areas Mol Biol. 1978;46:33–203. doi: 10.1002/9780470122914.ch2. [DOI] [PubMed] [Google Scholar]
- Hanson J. W., Dailey H. A. Purification and characterization of chicken erythrocyte ferrochelatase. Biochem J. 1984 Sep 15;222(3):695–700. doi: 10.1042/bj2220695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton J. D., Honeybourne C. L., Smith K. M., Tabba H. D., Jones O. T. The use of N-methylprotoporphyrin dimethyl ester to inhibit ferrochelatase in Rhodopseudomonas sphaeroides and its effect in promoting biosynthesis of magnesium tetrapyrroles. Biochem J. 1982 Nov 15;208(2):479–486. doi: 10.1042/bj2080479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. S., Jones O. T. Ferrochelatase of Rhodopseudomonas spheroides. Biochem J. 1970 Sep;119(3):453–462. doi: 10.1042/bj1190453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPorte D. C., Walsh K., Koshland D. E., Jr The branch point effect. Ultrasensitivity and subsensitivity to metabolic control. J Biol Chem. 1984 Nov 25;259(22):14068–14075. [PubMed] [Google Scholar]
- Lascelles J. The regulation of haem and chlorophyll synthesis. Biochem Soc Symp. 1968;28:49–59. [PubMed] [Google Scholar]
- Moody M. D., Dailey H. A. Iron transport and its relation to heme biosynthesis in Rhodopseudomonas sphaeroides. J Bacteriol. 1985 Mar;161(3):1074–1079. doi: 10.1128/jb.161.3.1074-1079.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody M. D., Dailey H. A. Siderophore utilization and iron uptake by Rhodopseudomonas sphaeroides. Arch Biochem Biophys. 1984 Oct;234(1):178–186. doi: 10.1016/0003-9861(84)90339-4. [DOI] [PubMed] [Google Scholar]
- Ortiz de Montellano P. R., Kunze K. L., Cole S. P., Marks G. S. Inhibition of hepatic ferrochelatase by the four isomers of N-methylprotoporphyrin IX. Biochem Biophys Res Commun. 1980 Dec 31;97(4):1436–1442. doi: 10.1016/s0006-291x(80)80026-x. [DOI] [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Perutz M. F. Regulation of oxygen affinity of hemoglobin: influence of structure of the globin on the heme iron. Annu Rev Biochem. 1979;48:327–386. doi: 10.1146/annurev.bi.48.070179.001551. [DOI] [PubMed] [Google Scholar]
- Riordan J. F. Functional arginyl residues in carboxypeptidase A. Modification with butanedione. Biochemistry. 1973 Sep 25;12(20):3915–3923. doi: 10.1021/bi00744a020. [DOI] [PubMed] [Google Scholar]
- Taketani S., Tokunaga R. Rat liver ferrochelatase. Purification, properties, and stimulation by fatty acids. J Biol Chem. 1981 Dec 25;256(24):12748–12753. [PubMed] [Google Scholar]
- Williams J. W., Morrison J. F. The kinetics of reversible tight-binding inhibition. Methods Enzymol. 1979;63:437–467. doi: 10.1016/0076-6879(79)63019-7. [DOI] [PubMed] [Google Scholar]


