Abstract
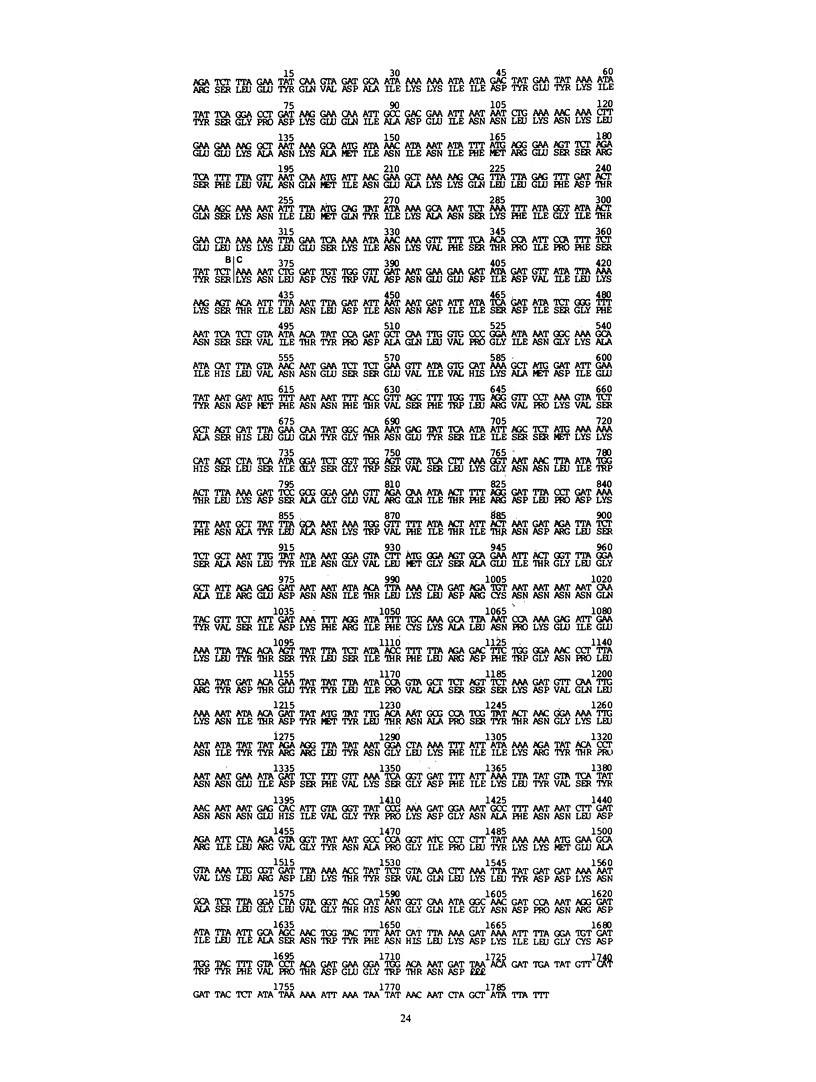
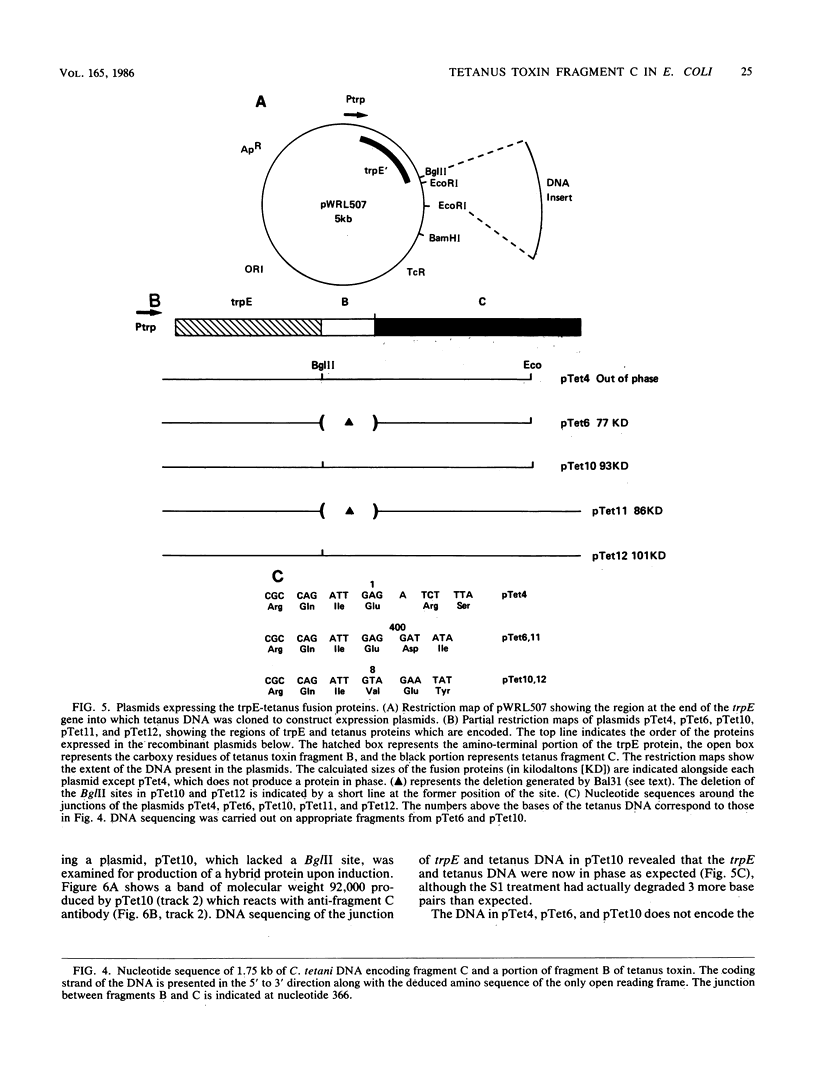
The amino acid sequence of the first 30 residues of fragment C of tetanus toxin was determined, and a mixture of 32 complementary oligonucleotides, each 17 bases long, was synthesized. A 2-kilobase (kb) EcoI fragment of Clostridium tetani DNA was identified by Southern blotting and was cloned into the Escherichia coli plasmid vector pAT153 with the 32P-labeled oligonucleotide mixture as a probe. A second 3.2-kb Bg/II fragment was identified and cloned with the 2-kb EcoRI fragment as a probe. The nucleotide sequence of 1.8 kb of this DNA was determined and was shown to encode the entire fragment C and a portion of fragment B of tetanus toxin. The tetanus DNA was expressed in E. coli with pWRL507, a plasmid vector containing the trp promoter and a portion of the trpE gene. The trpE-tetanus fusion proteins were visualized by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and were shown to react with anti-fragment C antibody.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bizzini B. Tetanus toxin. Microbiol Rev. 1979 Jun;43(2):224–240. doi: 10.1128/mr.43.2.224-240.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boquet P., Duflot E. Tetanus toxin fragment forms channels in lipid vesicles at low pH. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7614–7618. doi: 10.1073/pnas.79.24.7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidels L., Proia R. L., Hart D. A. Membrane receptors for bacterial toxins. Microbiol Rev. 1983 Dec;47(4):596–620. doi: 10.1128/mr.47.4.596-620.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairweather N., Kennedy S., Foster T. J., Kehoe M., Dougan G. Expression of a cloned Staphylococcus aureus alpha-hemolysin determinant in Bacillus subtilis and Staphylococcus aureus. Infect Immun. 1983 Sep;41(3):1112–1117. doi: 10.1128/iai.41.3.1112-1117.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn C. W., Jr, Silver R. P., Habig W. H., Hardegree M. C., Zon G., Garon C. F. The structural gene for tetanus neurotoxin is on a plasmid. Science. 1984 May 25;224(4651):881–884. doi: 10.1126/science.6326263. [DOI] [PubMed] [Google Scholar]
- Helting T. B., Nau H. H. Analysis of the immune response to papain digestion products of tetanus toxin. Acta Pathol Microbiol Immunol Scand C. 1984 Feb;92(1):59–63. doi: 10.1111/j.1699-0463.1984.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Helting T. B., Ronneberger H. J., Vollerthun R., Neubauer V. Toxicity of papain-digested tetanus toxin. Pathological effect of fragment B in the absence of spastic paralysis. J Biol Chem. 1978 Jan 10;253(1):125–129. [PubMed] [Google Scholar]
- Helting T. B., Zwisler O. Structure of tetanus toxin. I. Breakdown of the toxin molecule and discrimination between polypeptide fragments. J Biol Chem. 1977 Jan 10;252(1):187–193. [PubMed] [Google Scholar]
- Helting T. B., Zwisler O., Wiegandt H. Structure of tetanus toxin. II. Toxin binding to ganglioside. J Biol Chem. 1977 Jan 10;252(1):194–198. [PubMed] [Google Scholar]
- KIMMEL J. R., SMITH E. L. Crystalline papain. I. Preparation, specificity, and activation. J Biol Chem. 1954 Apr;207(2):515–531. [PubMed] [Google Scholar]
- Kenimer J. G., Habig W. H., Hardegree M. C. Monoclonal antibodies as probes of tetanus toxin structure and function. Infect Immun. 1983 Dec;42(3):942–948. doi: 10.1128/iai.42.3.942-948.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M., Yoneda M. Isolation and purification of two antigenically active, "complimentary" polypeptide fragments of tetanus neurotoxin. Infect Immun. 1975 Nov;12(5):1147–1153. doi: 10.1128/iai.12.5.1147-1153.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer V., Helting T. B. Structure of tetanus toxin. N-Terminal amino acid analysis of the two molecular forms of tetanus toxin and its composite chains. Biochem Biophys Res Commun. 1979 Feb 14;86(3):635–642. doi: 10.1016/0006-291x(79)91760-1. [DOI] [PubMed] [Google Scholar]
- Nichols B. P., Yanofsky C. Plasmids containing the trp promoters of Escherichia coli and Serratia marcescens and their use in expressing cloned genes. Methods Enzymol. 1983;101:155–164. doi: 10.1016/0076-6879(83)01011-3. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Odink K. G., Lockyer M. J., Nicholls S. C., Hillman Y., Freeman R. R., Holder A. A. Expression of cloned cDNA for a major surface antigen of Plasmodium falciparum merozoites. FEBS Lett. 1984 Jul 23;173(1):108–112. doi: 10.1016/0014-5793(84)81027-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Volk W. A., Bizzini B., Snyder R. M., Bernhard E., Wagner R. R. Neutralization of tetanus toxin by distinct monoclonal antibodies binding to multiple epitopes on the toxin molecule. Infect Immun. 1984 Sep;45(3):604–609. doi: 10.1128/iai.45.3.604-609.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. B., Johnson M. J., Hirose T., Miyake T., Kawashima E. H., Itakura K. The use of synthetic oligonucleotides as hybridization probes. II. Hybridization of oligonucleotides of mixed sequence to rabbit beta-globin DNA. Nucleic Acids Res. 1981 Feb 25;9(4):879–894. doi: 10.1093/nar/9.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C., Platt T., Crawford I. P., Nichols B. P., Christie G. E., Horowitz H., VanCleemput M., Wu A. M. The complete nucleotide sequence of the tryptophan operon of Escherichia coli. Nucleic Acids Res. 1981 Dec 21;9(24):6647–6668. doi: 10.1093/nar/9.24.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heyningen S. Binding of ganglioside by the chains of tetanus toxin. FEBS Lett. 1976 Sep 15;68(1):5–7. doi: 10.1016/0014-5793(76)80391-2. [DOI] [PubMed] [Google Scholar]




