Abstract
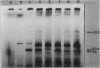
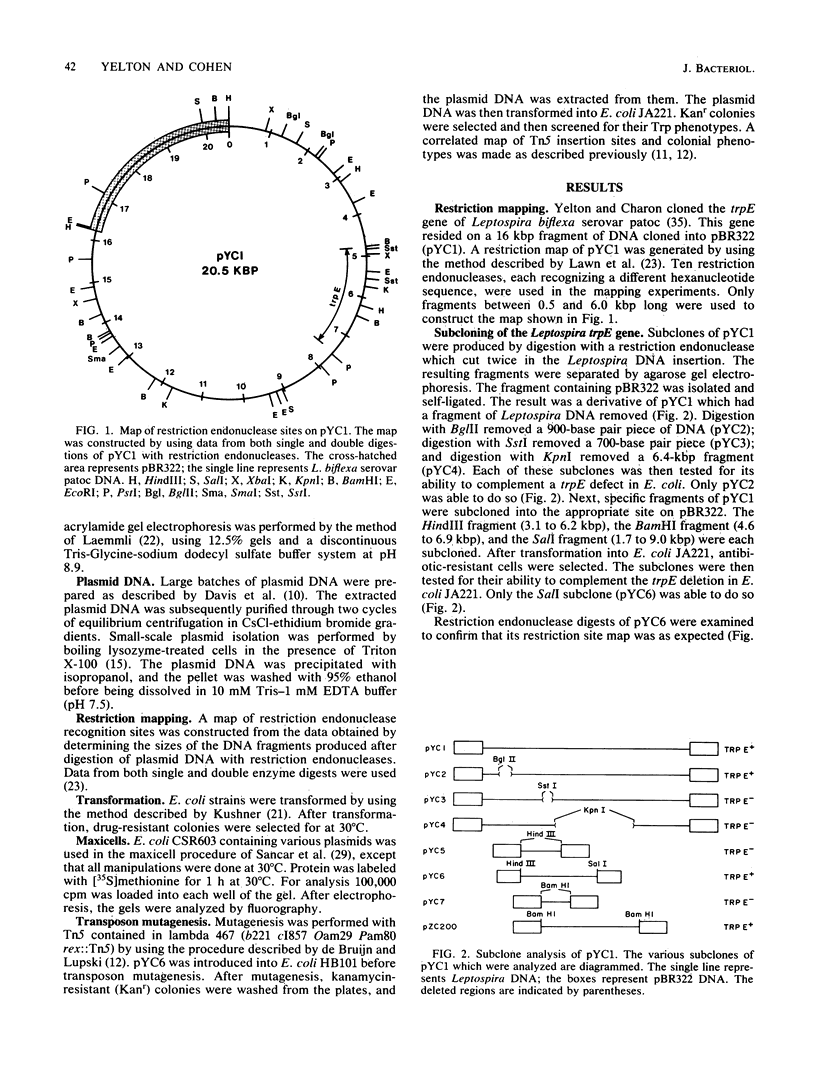
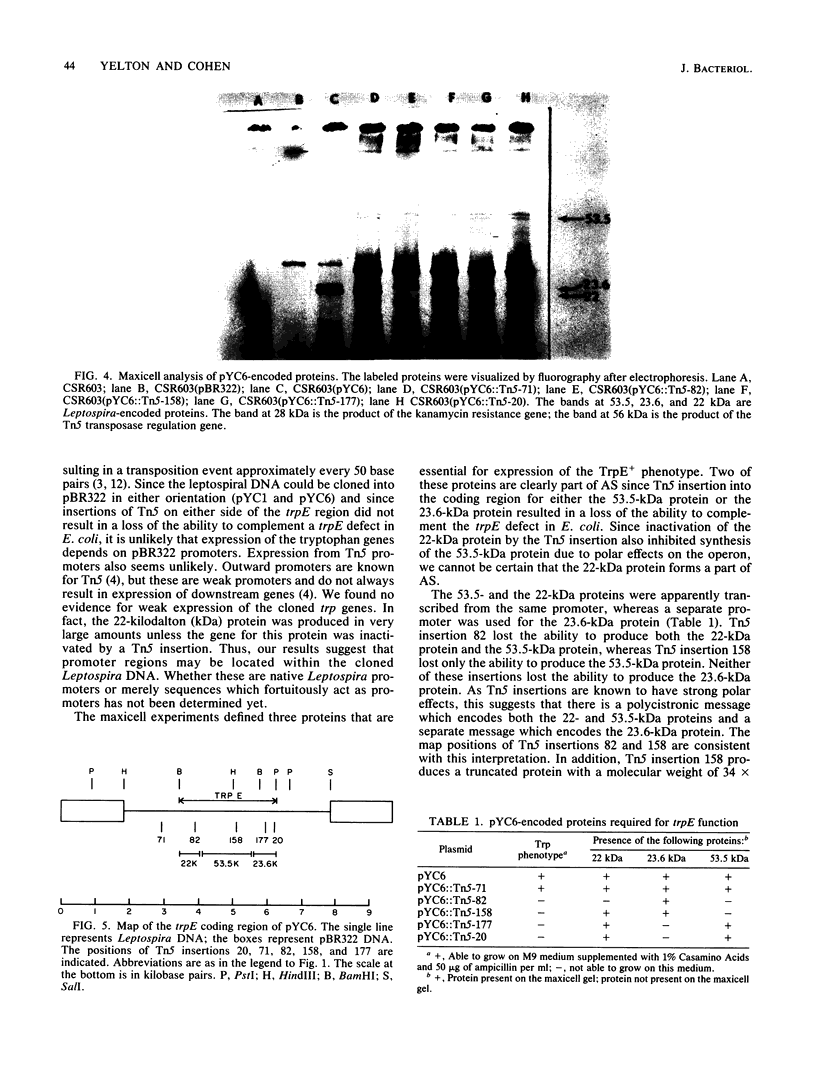
To analyze the cloned region of the chromosome of the spirochete Leptospira biflexa serovar patoc which complemented a defect in the trpE gene of Escherichia coli, we performed a series of experiments involving subcloning, transposon mutagenesis, and maxicells. By subcloning into pBR322 we were able to isolate the Leptospira genes on a 9.7-kilobase pair plasmid (pYC6). Transposon mutagenesis with Tn5 identified a 2.8-kilobase pair region of this plasmid as being necessary to complement a trpE deletion mutation in E. coli. Transformation of plasmid pYC6 into E. coli cells deleted for trpE and the proximal end of trpD showed that the Leptospira DNA complemented both defects. A maxicell analysis of various transposon-induced mutations of the plasmid revealed that three proteins (53.5, 23.6, and 22 kilodaltons) were encoded by the 2.8-kilobase pair region of the Leptospira genome. Two different promoters controlled the production of these three proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Band L., Shimotsu H., Henner D. J. Nucleotide sequence of the Bacillus subtilis trpE and trpD genes. Gene. 1984 Jan;27(1):55–65. doi: 10.1016/0378-1119(84)90238-5. [DOI] [PubMed] [Google Scholar]
- Baseman J. B., Cox C. D. Intermediate energy metabolism of Leptospira. J Bacteriol. 1969 Mar;97(3):992–1000. doi: 10.1128/jb.97.3.992-1000.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. E., Schmandt M. A., Lowe J. B. Specificity of transposon Tn5 insertion. Genetics. 1983 Dec;105(4):813–828. doi: 10.1093/genetics/105.4.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. E., Weiss A., Crossland L. Polarity of Tn5 insertion mutations in Escherichia coli. J Bacteriol. 1980 May;142(2):439–446. doi: 10.1128/jb.142.2.439-446.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun D. H., Pierson D. L., Jensen R. A. The regulation of tryptophan biosynthesis in Pseudomonas aeruginosa. Mol Gen Genet. 1973 Mar 1;121(2):117–132. doi: 10.1007/BF00277526. [DOI] [PubMed] [Google Scholar]
- Charon N. W., Johnson R. C., Peterson D. Amino acid biosynthesis in the spirochete Leptospira: evidence for a novel pathway of isoleucine biosynthesis. J Bacteriol. 1974 Jan;117(1):203–211. doi: 10.1128/jb.117.1.203-211.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinault A. C., Carbon J. Overlap hybridization screening: isolation and characterization of overlapping DNA fragments surrounding the leu2 gene on yeast chromosome III. Gene. 1979 Feb;5(2):111–126. doi: 10.1016/0378-1119(79)90097-0. [DOI] [PubMed] [Google Scholar]
- Crawford I. P. Comparative studies on the regulation of tryptophan synthesis. CRC Crit Rev Biochem. 1980;8(2):175–189. doi: 10.3109/10409238009105468. [DOI] [PubMed] [Google Scholar]
- Crawford I. P. Gene rearrangements in the evolution of the tryptophan synthetic pathway. Bacteriol Rev. 1975 Jun;39(2):87–120. doi: 10.1128/br.39.2.87-120.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox G. E., Stackebrandt E., Hespell R. B., Gibson J., Maniloff J., Dyer T. A., Wolfe R. S., Balch W. E., Tanner R. S., Magrum L. J. The phylogeny of prokaryotes. Science. 1980 Jul 25;209(4455):457–463. doi: 10.1126/science.6771870. [DOI] [PubMed] [Google Scholar]
- Henneberry R. C., Cox C. D. Beta-oxidation of fatty acids by Leptospira. Can J Microbiol. 1970 Jan;16(1):41–45. doi: 10.1139/m70-007. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Holt S. C. Anatomy and chemistry of spirochetes. Microbiol Rev. 1978 Mar;42(1):114–160. doi: 10.1128/mr.42.1.114-160.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz H., Van Arsdell J., Platt T. Nucleotide sequence of the trpD and trpC genes of Salmonella typhimurium. J Mol Biol. 1983 Oct 5;169(4):775–797. doi: 10.1016/s0022-2836(83)80136-3. [DOI] [PubMed] [Google Scholar]
- Jackson E. N., Yanofsky C. Localization of two functions of the phosphoribosyl anthranilate transferase of Escherichia coli to distinct regions of the polypeptide chain. J Bacteriol. 1974 Feb;117(2):502–508. doi: 10.1128/jb.117.2.502-508.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C. The spirochetes. Annu Rev Microbiol. 1977;31:89–106. doi: 10.1146/annurev.mi.31.100177.000513. [DOI] [PubMed] [Google Scholar]
- Kaplan J. B., Goncharoff P., Seibold A. M., Nichols B. P. Nucleotide sequence of the Acinetobacter calcoaceticus trpGDC gene cluster. Mol Biol Evol. 1984 Nov;1(6):456–472. doi: 10.1093/oxfordjournals.molbev.a040331. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Miozzari G. F., Yanofsky C. Gene fusion during the evolution of the tryptophan operon in enterobacteriaceae. Nature. 1979 Feb 8;277(5696):486–489. doi: 10.1038/277486a0. [DOI] [PubMed] [Google Scholar]
- Queener S. F., Gunsalus I. C. Anthranilate synthase enzyme system and complementation in Pseudomonas species. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1225–1232. doi: 10.1073/pnas.67.3.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall H. N., Charon N. W., Peterson D. E. Multiple pathways for isoleucine biosynthesis in the spirochete Leptospira. J Bacteriol. 1983 May;154(2):846–853. doi: 10.1128/jb.154.2.846-853.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler M. E., Schoenlein P. V., Ross C. M., Barrett J. T., Ely B. Genetic and physical analyses of Caulobacter crescentus trp genes. J Bacteriol. 1984 Oct;160(1):279–287. doi: 10.1128/jb.160.1.279-287.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C. Attenuation in the control of expression of bacterial operons. Nature. 1981 Feb 26;289(5800):751–758. doi: 10.1038/289751a0. [DOI] [PubMed] [Google Scholar]
- Yanofsky C. Comparison of regulatory and structural regions of genes of tryptophan metabolism. Mol Biol Evol. 1984 Feb;1(2):143–161. doi: 10.1093/oxfordjournals.molbev.a040307. [DOI] [PubMed] [Google Scholar]
- Yanofsky C., Platt T., Crawford I. P., Nichols B. P., Christie G. E., Horowitz H., VanCleemput M., Wu A. M. The complete nucleotide sequence of the tryptophan operon of Escherichia coli. Nucleic Acids Res. 1981 Dec 21;9(24):6647–6668. doi: 10.1093/nar/9.24.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelton D. B., Charon N. W. Cloning of a gene required for tryptophan biosynthesis from Leptospira biflexa serovar patoc into Escherichia coli. Gene. 1984 May;28(2):147–152. doi: 10.1016/0378-1119(84)90251-8. [DOI] [PubMed] [Google Scholar]
- Zalkin H. Anthranilate synthetase. Adv Enzymol Relat Areas Mol Biol. 1973;38:1–39. doi: 10.1002/9780470122839.ch1. [DOI] [PubMed] [Google Scholar]
- de Bruijn F. J., Ausubel F. M. The cloning and transposon Tn5 mutagenesis of the glnA region of Klebsiella pneumoniae: identification of glnR, a gene involved in the regulation of the nif and hut operons. Mol Gen Genet. 1981;183(2):289–297. doi: 10.1007/BF00270631. [DOI] [PubMed] [Google Scholar]
- de Bruijn F. J., Lupski J. R. The use of transposon Tn5 mutagenesis in the rapid generation of correlated physical and genetic maps of DNA segments cloned into multicopy plasmids--a review. Gene. 1984 Feb;27(2):131–149. doi: 10.1016/0378-1119(84)90135-5. [DOI] [PubMed] [Google Scholar]