Abstract
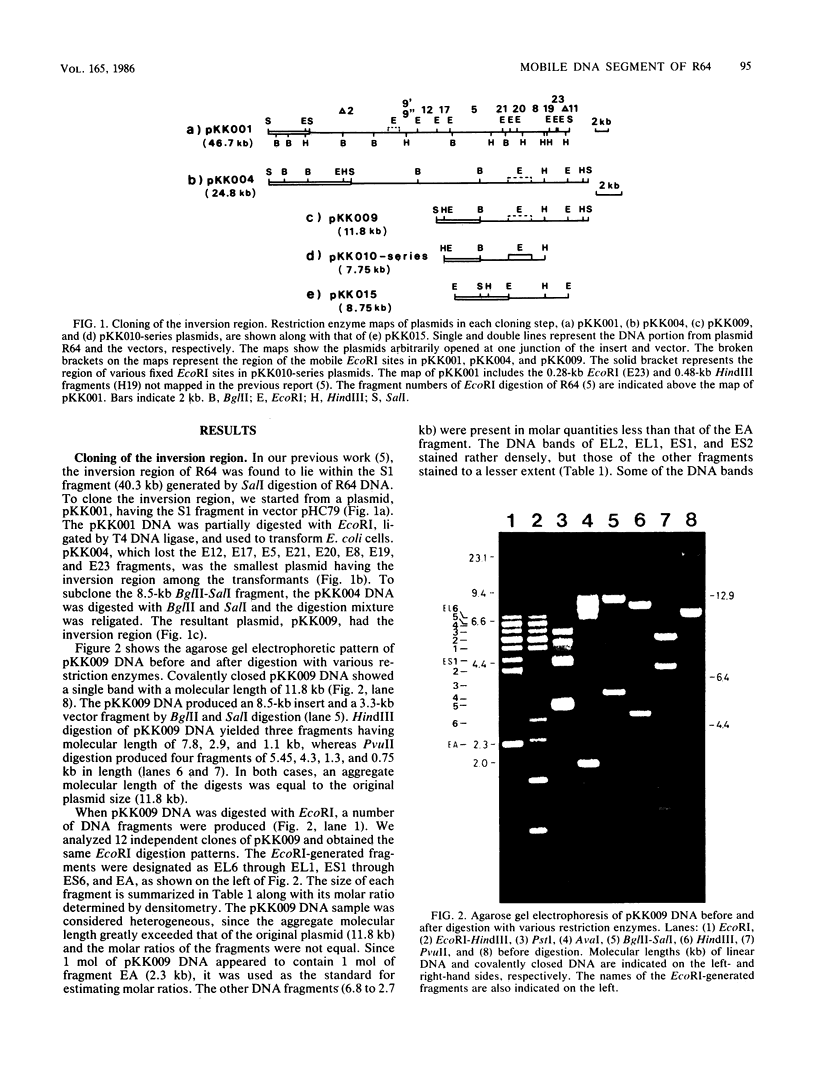
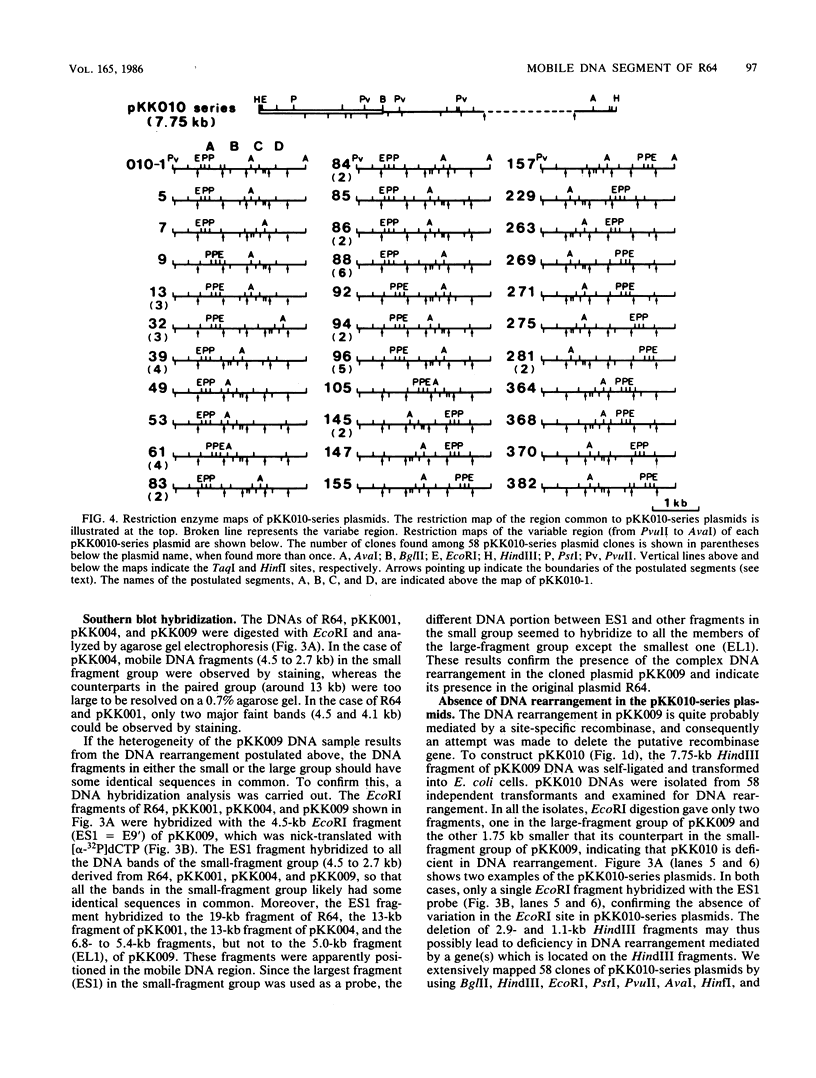
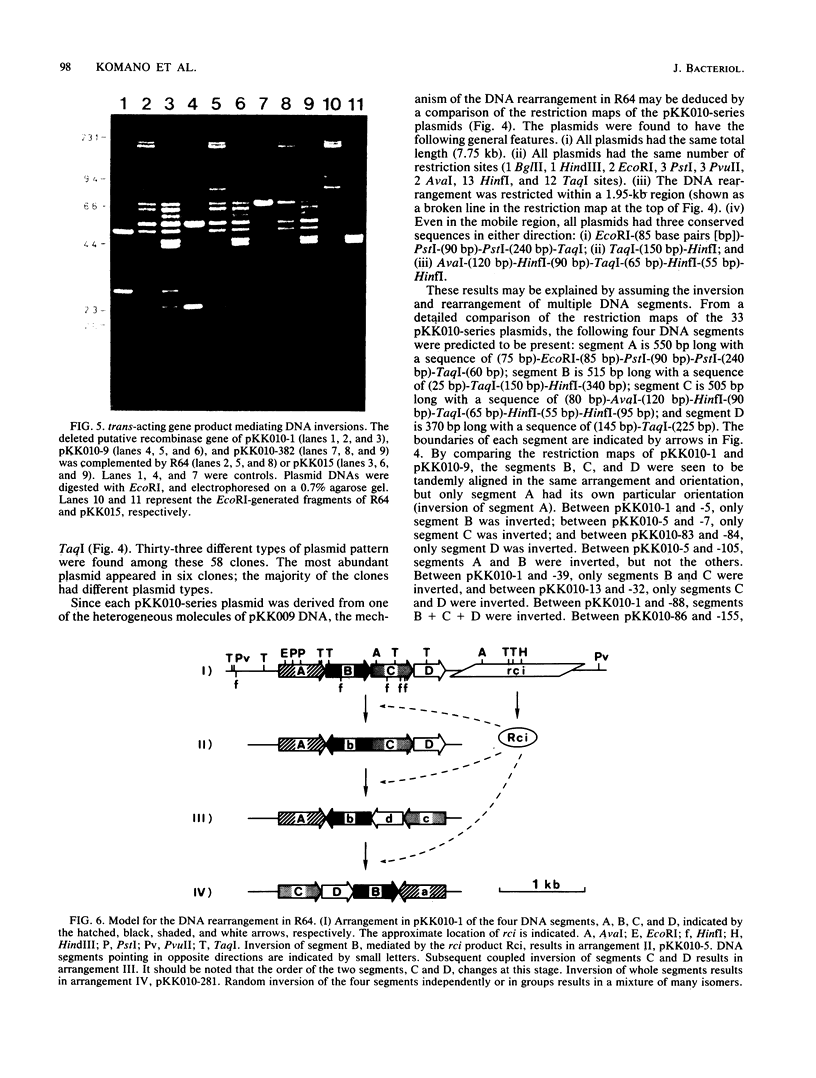
When R64 DNA was digested with EcoRI, two DNA fragments not equimolar to the plasmid DNA were produced. A DNA region including these fragments was cloned (pKK009), and the pKK009 DNA sample was found to be a mixture of six or more DNA species with EcoRI, PstI, and AvaI cleavage sites at different positions, suggesting a complex rearrangement of DNA. When a part of the pKK009 DNA was removed by HindIII digestion, 33 different types of plasmids (pKK010-series plasmids) were obtained out of 58 clones tested, but no DNA rearrangement could be observed. On the basis of a comparison of the detailed restriction maps of these pKK010-series plasmids, we propose a model in which four DNA segments invert independently or in groups within the 1.95-kilobase region of R64, so that the arrangements of these four segments change randomly. The fixed pKK010-series plasmid DNA was again rearranged in the presence of R64, indicating that trans-acting gene function may be present to mediate the DNA rearrangement. The gene (tentatively designated as rci) was located on a 4.5-kilobase E9' fragment of R64.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari A. I., Ambrosio L. The invertible segment of bacteriophage Mu DNA determines the adsorption properties of Mu particles. Nature. 1978 Feb 9;271(5645):575–577. doi: 10.1038/271575a0. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Carbon J. Functional expression of cloned yeast DNA in Escherichia coli: specific complementation of argininosuccinate lyase (argH) mutations. J Mol Biol. 1978 Apr 25;120(4):517–532. doi: 10.1016/0022-2836(78)90351-0. [DOI] [PubMed] [Google Scholar]
- Furuichi T., Komano T., Nisioka T. Physical and genetic analyses of the Inc-I alpha plasmid R64. J Bacteriol. 1984 Jun;158(3):997–1004. doi: 10.1128/jb.158.3.997-1004.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiestand-Nauer R., Iida S. Sequence of the site-specific recombinase gene cin and of its substrates serving in the inversion of the C segment of bacteriophage P1. EMBO J. 1983;2(10):1733–1740. doi: 10.1002/j.1460-2075.1983.tb01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Kamp D., Kahmann R., Zipser D., Broker T. R., Chow L. T. Inversion of the G DNA segment of phage Mu controls phage infectivity. Nature. 1978 Feb 9;271(5645):577–580. doi: 10.1038/271577a0. [DOI] [PubMed] [Google Scholar]
- Kutsukake K., Iino T. Inversions of specific DNA segments in flagellar phase variation of Salmonella and inversion systems of bacteriophages P1 and Mu. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7338–7341. doi: 10.1073/pnas.77.12.7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. J., Otsubo E., Deonier R. C., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. V. ilv+ Deletion mutants of F14. J Mol Biol. 1974 Nov 15;89(4):585–597. doi: 10.1016/0022-2836(74)90037-0. [DOI] [PubMed] [Google Scholar]
- Mertens G., Hoffmann A., Blöcker H., Frank R., Kahmann R. Gin-mediated site-specific recombination in bacteriophage Mu DNA: overproduction of the protein and inversion in vitro. EMBO J. 1984 Oct;3(10):2415–2421. doi: 10.1002/j.1460-2075.1984.tb02148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E., Novick R. P. Physical mapping of Staphylococcus aureus penicillinase plasmid pI524: characterization of an invertible region. Mol Gen Genet. 1979 Aug;175(1):19–30. doi: 10.1007/BF00267851. [DOI] [PubMed] [Google Scholar]
- Plasterk R. H., Brinkman A., van de Putte P. DNA inversions in the chromosome of Escherichia coli and in bacteriophage Mu: relationship to other site-specific recombination systems. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5355–5358. doi: 10.1073/pnas.80.17.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plasterk R. H., Van de Putte P. Genetic switches by DNA inversions in prokaryotes. Biochim Biophys Acta. 1984 Jun 16;782(2):111–119. doi: 10.1016/0167-4781(84)90013-7. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roizman B. The structure and isomerization of herpes simplex virus genomes. Cell. 1979 Mar;16(3):481–494. doi: 10.1016/0092-8674(79)90023-0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Symonds N., Coelho A. Role of the G segment in the growth of phage Mu. Nature. 1978 Feb 9;271(5645):573–574. doi: 10.1038/271573a0. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Zieg J., Silverman M., Hilmen M., Simon M. Recombinational switch for gene expression. Science. 1977 Apr 8;196(4286):170–172. doi: 10.1126/science.322276. [DOI] [PubMed] [Google Scholar]
- Zieg J., Simon M. Analysis of the nucleotide sequence of an invertible controlling element. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4196–4200. doi: 10.1073/pnas.77.7.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]