Abstract
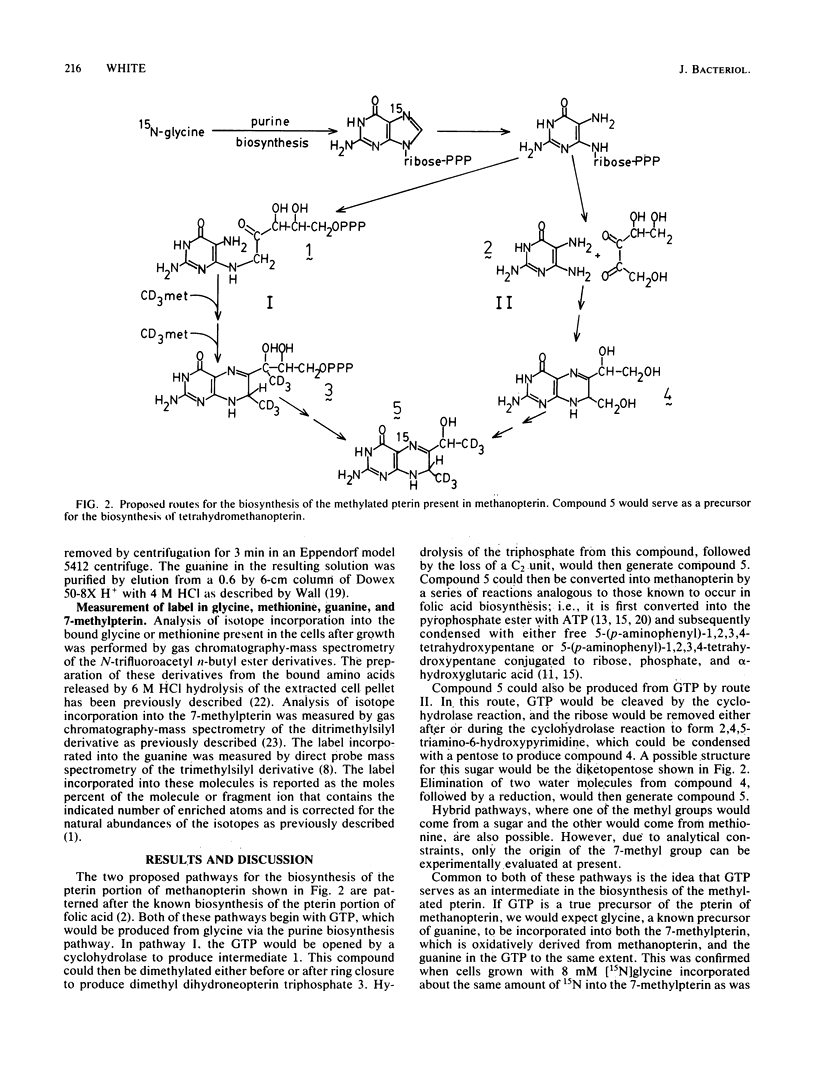
The incorporation of [15N]glycine and [U-methyl-2H]methionine into methanopterin by growing cells of a methanogenic bacterium was measured to establish the biosynthetic route of the methylated pterin in the structure. The tetrahydromethanopterin produced by the cells was oxidatively cleaved to produce 7-methylpterin, and the amount of label incorporated into this pterin was measured by gas chromatography-mass spectrometry of the ditrimethylsilyl derivative of this compound. Approximately 27% of the 7-methylpterin and the guanine present in the cell was derived from the fed [15N]glycine. [U-methyl-2H]methionine was incorporated with the initial retention of all three deuteriums. These results are consistent with the biosynthesis of the pterin of methanopterin originating from GTP and its 7-methyl group arising from the methyl group of methionine.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ekiel I., Smith I. C., Sprott G. D. Biosynthetic pathways in Methanospirillum hungatei as determined by 13C nuclear magnetic resonance. J Bacteriol. 1983 Oct;156(1):316–326. doi: 10.1128/jb.156.1.316-326.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante-Semerena J. C., Leigh J. A., Rinehart K. L., Wolfe R. S. Formaldehyde activation factor, tetrahydromethanopterin, a coenzyme of methanogenesis. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1976–1980. doi: 10.1073/pnas.81.7.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante-Semerena J. C., Rinehart K. L., Jr, Wolfe R. S. Tetrahydromethanopterin, a carbon carrier in methanogenesis. J Biol Chem. 1984 Aug 10;259(15):9447–9455. [PubMed] [Google Scholar]
- Escalante-Semerena J. C., Wolfe R. S. Tetrahydromethanopterin-dependent methanogenesis from non-physiological C1 donors in Methanobacterium thermoautotrophicum. J Bacteriol. 1985 Feb;161(2):696–701. doi: 10.1128/jb.161.2.696-701.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrke C. W., Ruyle C. D. Gas-liquid chromatographic analysis of nucleic acid components. J Chromatogr. 1968 Dec 17;38(4):473–491. doi: 10.1016/0021-9673(68)85076-9. [DOI] [PubMed] [Google Scholar]
- Lovley D. R., Greening R. C., Ferry J. G. Rapidly growing rumen methanogenic organism that synthesizes coenzyme M and has a high affinity for formate. Appl Environ Microbiol. 1984 Jul;48(1):81–87. doi: 10.1128/aem.48.1.81-87.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz P. J., Hotchkiss R. D. The enzymatic synthesis of dihydrofolate and dihydropteroate in cell-free preparations from wild-type and sulfonamide-resistant pneumococcus. Biochemistry. 1966 Jan;5(1):67–74. doi: 10.1021/bi00865a010. [DOI] [PubMed] [Google Scholar]
- Richey D. P., Brown G. M. The biosynthesis of folic acid. IX. Purification and properties of the enzymes required for the formation of dihydropteroic acid. J Biol Chem. 1969 Mar 25;244(6):1582–1592. [PubMed] [Google Scholar]
- SHIOTA T., DISRAELY M. N., MCCANN M. P. THE ENZYMATIC SYNTHESIS OF FOLATE-LIKE COMPOUNDS FROM HYDROXYMETHYLDIHYDROPTERIDINE PYROPHOSPHATE. J Biol Chem. 1964 Jul;239:2259–2266. [PubMed] [Google Scholar]
- Shiota T., Baugh C. M., Jackson R., Dillard R. The enzymatic synthesis of hydroxymethyldihydropteridine pyrophosphate and dihydrofolate. Biochemistry. 1969 Dec;8(12):5022–5028. doi: 10.1021/bi00840a052. [DOI] [PubMed] [Google Scholar]
- WEISMAN R. A., BROWN G. M. THE BIOSYNTHESIS OF FOLIC ACID. V. CHARACTERISTICS OF THE ENZYME SYSTEM THAT CATALYZES THE SYNTHESIS OF DIHYDROPTEROIC ACID. J Biol Chem. 1964 Jan;239:326–331. [PubMed] [Google Scholar]
- White R. H. 7-Methylpterin and 7-methyllumizine: oxidative degradation products of 7-methyl-substituted pteridines in methanogenic bacteria. J Bacteriol. 1985 May;162(2):516–520. doi: 10.1128/jb.162.2.516-520.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. H. A method for the measurement of sulfur-34 abundance in bound cysteine and methionine. Anal Biochem. 1981 Jul 1;114(2):349–354. doi: 10.1016/0003-2697(81)90492-9. [DOI] [PubMed] [Google Scholar]
- van Beelen P., Labro J. F., Keltjens J. T., Geerts W. J., Vogels G. D., Laarhoven W. H., Guijt W., Haasnoot C. A. Derivatives of methanopterin, a coenzyme involved in methanogenesis. Eur J Biochem. 1984 Mar 1;139(2):359–365. doi: 10.1111/j.1432-1033.1984.tb08014.x. [DOI] [PubMed] [Google Scholar]
- van Beelen P., Stassen A. P., Bosch J. W., Vogels G. D., Guijt W., Haasnoot C. A. Elucidation of the structure of methanopterin, a coenzyme from Methanobacterium thermoautotrophicum, using two-dimensional nuclear-magnetic-resonance techniques. Eur J Biochem. 1984 Feb 1;138(3):563–571. doi: 10.1111/j.1432-1033.1984.tb07951.x. [DOI] [PubMed] [Google Scholar]


