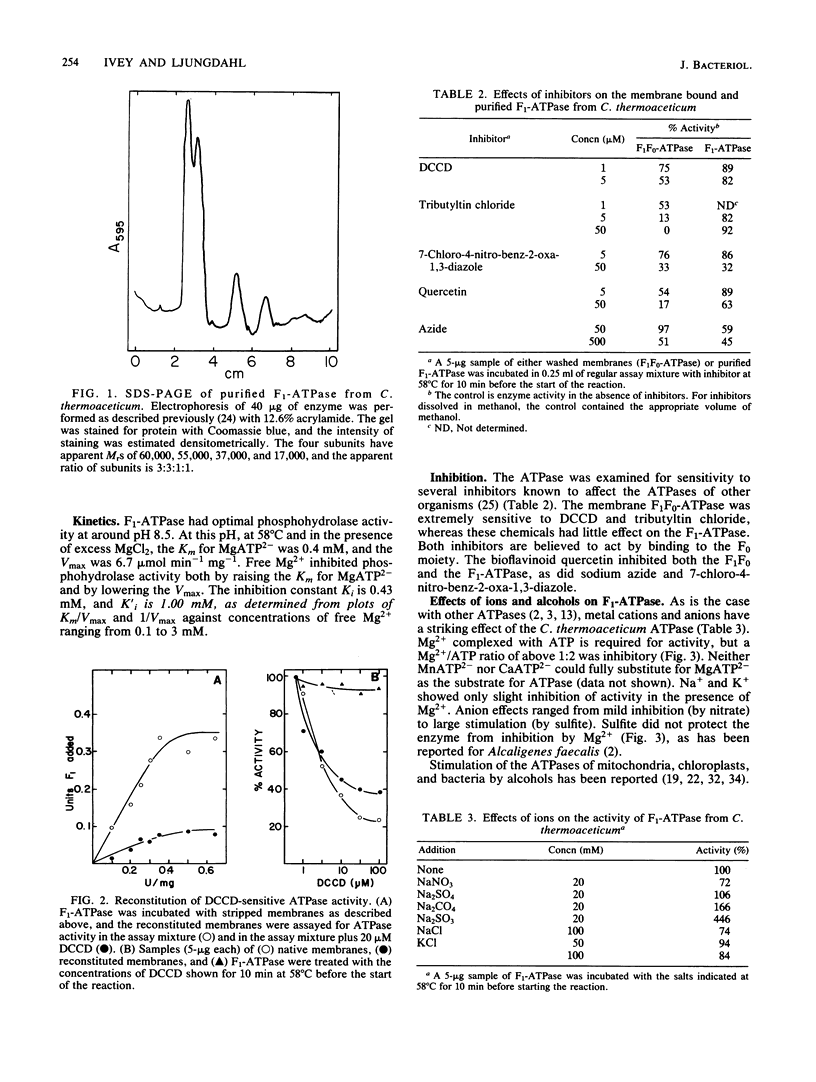
Abstract
The F1 portion of the H+-ATPase from Clostridium thermoaceticum was purified to homogeneity by solubilization at low ionic strength, ion-exchange chromatography, and gel filtration. The last indicated the Mr to be 370,000. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the pure enzyme revealed four bands with Mr corresponding to 60,000, 55,000, 37,000, and 17,000 in an apparent molar ratio of 3:3:1:1. The purified enzyme would bind to stripped membranes to reconstitute dicyclohexylcarbodiimide-sensitive ATPase activity. Phosphohydrolase activity, measured at 58 degrees C, was optimal at pH 8.5. In the presence of a 1 mM excess of Mg2+ over the concentration of ATP, the Km for ATP was 0.4 mM, and the Vmax was 6.7 mumol min-1 mg-1. Unlike the membrane-bound F1F0 complex, the F1-ATPase was relatively insensitive to the inhibitors dicyclohexylcarbodiimide and tributyltin chloride. Both the complex and the F1-ATPase were inhibited by quercetin, azide, 7-chloro-4-nitro-benz-2-oxa-1,3-diazole, and free magnesium, and both were stimulated by primary alcohols and sulfite. In whole cells, the F1F0-ATPase catalyzed the synthesis of ATP in response to a pH gradient.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adolfsen R., Moudrianakis E. N. Control of complex metal ion equilibria in biochemical reaction systems. Intrinsic and apparent stability constants of metal-adenine nucleotide complexes. J Biol Chem. 1978 Jun 25;253(12):4378–4379. [PubMed] [Google Scholar]
- Adolfsen R., Moudrianakis E. N. Kinetic mechanisms of ionic activation and inhibition of the adenosine triphosphatase of the 13 S coupling factor of oxidative phosphorylation. J Biol Chem. 1978 Jun 25;253(12):4380–4388. [PubMed] [Google Scholar]
- Ahlers J., Günther T. Kinetics of the ion-sensitive Mg, Ca-adenosinetriphosphatase (ATPase) from Escherichia coli. Arch Biochem Biophys. 1975 Nov;171(1):163–169. doi: 10.1016/0003-9861(75)90019-3. [DOI] [PubMed] [Google Scholar]
- Andreesen J. R., Schaupp A., Neurauter C., Brown A., Ljungdahl L. G. Fermentation of glucose, fructose, and xylose by Clostridium thermoaceticum: effect of metals on growth yield, enzymes, and the synthesis of acetate from CO 2 . J Bacteriol. 1973 May;114(2):743–751. doi: 10.1128/jb.114.2.743-751.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baginski E. S., Epstein E., Zak B. Review of phosphate methodologies. Ann Clin Lab Sci. 1975 Sep-Oct;5(5):399–416. [PubMed] [Google Scholar]
- Barker H. A., Kamen M. D. Carbon Dioxide Utilization in the Synthesis of Acetic Acid by Clostridium Thermoaceticum. Proc Natl Acad Sci U S A. 1945 Aug;31(8):219–225. doi: 10.1073/pnas.31.8.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beechey R. B., Hubbard S. A., Linnett P. E., Mitchell A. D., Munn E. A. A simple and rapid method for the preparation of adenosine triphosphatase from submitochondrial particles. Biochem J. 1975 Jun;148(3):533–537. doi: 10.1042/bj1480533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer J. M., Ashworth R. B. Disc electrophoresis. J Chem Educ. 1969 Jan;46(1):41–45. doi: 10.1021/ed046p41. [DOI] [PubMed] [Google Scholar]
- Clarke D. J., Fuller F. M., Morris J. G. The proton-translocating adenosine triphosphatase of the obligately anaerobic bacterium Clostridium pasteurianum. 1. ATP phosphohydrolase activity. Eur J Biochem. 1979 Aug 1;98(2):597–612. doi: 10.1111/j.1432-1033.1979.tb13222.x. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. Statistical analysis of enzyme kinetic data. Methods Enzymol. 1979;63:103–138. doi: 10.1016/0076-6879(79)63008-2. [DOI] [PubMed] [Google Scholar]
- Drake H. L. Demonstration of hydrogenase in extracts of the homoacetate-fermenting bacterium Clostridium thermoaceticum. J Bacteriol. 1982 May;150(2):702–709. doi: 10.1128/jb.150.2.702-709.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel R. E., Lardy H. A. Stimulation of rat liver mitochondrial adenosine triphosphatase by anions. J Biol Chem. 1975 Jan 10;250(1):191–196. [PubMed] [Google Scholar]
- Elliott J. I., Brewer J. M. The inactivation of yeast enolase by 2,3-butanedione. Arch Biochem Biophys. 1978 Sep;190(1):351–357. doi: 10.1016/0003-9861(78)90285-0. [DOI] [PubMed] [Google Scholar]
- Elliott J. I., Ljungdahl L. G. Isolation and characterization of an Fe,-S8 ferredoxin (ferredoxin II) from Clostridium thermoaceticum. J Bacteriol. 1982 Jul;151(1):328–333. doi: 10.1128/jb.151.1.328-333.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine F. E., Peterson W. H., McCoy E., Johnson M. J., Ritter G. J. A New Type of Glucose Fermentation by Clostridium thermoaceticum. J Bacteriol. 1942 Jun;43(6):701–715. doi: 10.1128/jb.43.6.701-715.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai M., Kanazawa H. Structure and function of proton-translocating adenosine triphosphatase (F0F1): biochemical and molecular biological approaches. Microbiol Rev. 1983 Sep;47(3):285–312. doi: 10.1128/mr.47.3.285-312.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwald M., Andreesen J. R., LeGall J., Ljungdahl L. G. Presence of cytochrome and menaquinone in Clostridium formicoaceticum and Clostridium thermoaceticum. J Bacteriol. 1975 Apr;122(1):325–328. doi: 10.1128/jb.122.1.325-328.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. Y., Senda M., Kanazawa H., Tsuchiya T., Futai M. Comparison of F1's of oxidative phosphorylation from Escherichia coli and Salmonella typhimurium and demonstration of interchangeability of their subunits. Biochemistry. 1984 Feb 28;23(5):988–993. doi: 10.1021/bi00300a029. [DOI] [PubMed] [Google Scholar]
- Kimmich G. A., Randles J., Brand J. S. Assay of picomole amounts of ATP, ADP, and AMP using the luciferase enzyme system. Anal Biochem. 1975 Nov;69(1):187–206. doi: 10.1016/0003-2697(75)90580-1. [DOI] [PubMed] [Google Scholar]
- Kohlbrenner W. E., Cross R. L. The mode of inhibition of oxidative phosphorylation by efrapeptin (A23871): measurement of substrate effects on rates of inactivation by a tight-binding inhibitor. Arch Biochem Biophys. 1979 Dec;198(2):598–607. doi: 10.1016/0003-9861(79)90536-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linnett P. E., Beechey R. B. Inhibitors of the ATP synthethase system. Methods Enzymol. 1979;55:472–518. doi: 10.1016/0076-6879(79)55061-7. [DOI] [PubMed] [Google Scholar]
- Ljungdahl L. G., Andreesen J. R. Formate dehydrogenase, a selenium--tungsten enzyme from Clostridium thermoaceticum. Methods Enzymol. 1978;53:360–372. doi: 10.1016/s0076-6879(78)53042-5. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Muñoz E. Polymorphism and conformational dynamics of F1-ATPases from bacterial membranes. A model for the regulation of these enzymes on the basis of molecular plasticity. Biochim Biophys Acta. 1982 May 12;650(4):233–265. doi: 10.1016/0304-4157(82)90018-1. [DOI] [PubMed] [Google Scholar]
- Nelson N., Nelson H., Racker E. Partial resolution of the enzymes catalyzing photophosphorylation. XI. Magnesium-adenosine triphosphatase properties of heat-activated coupling factor I from chloroplasts. J Biol Chem. 1972 Oct 25;247(20):6506–6510. [PubMed] [Google Scholar]
- Penefsky H. S., Warner R. C. Partial resolution of the enzymes catalyzing oxidative phosphorylation. VI. Studies on the mechanism of cold inactivation of mitochondrial adenosine triphosphatase. J Biol Chem. 1965 Dec;240(12):4694–4702. [PubMed] [Google Scholar]
- Sherman P. A., Wimmer M. J. Activation of ATPase of spinach coupling factor 1. Release of tightly bound ADP from the soluble enzyme. Eur J Biochem. 1984 Mar 1;139(2):367–371. doi: 10.1111/j.1432-1033.1984.tb08015.x. [DOI] [PubMed] [Google Scholar]
- WOOD H. G. A study of carbon dioxide fixation by mass determination of the types of C13-acetate. J Biol Chem. 1952 Feb;194(2):905–931. [PubMed] [Google Scholar]
- Yamamoto I., Saiki T., Liu S. M., Ljungdahl L. G. Purification and properties of NADP-dependent formate dehydrogenase from Clostridium thermoaceticum, a tungsten-selenium-iron protein. J Biol Chem. 1983 Feb 10;258(3):1826–1832. [PubMed] [Google Scholar]
- Yang S. S., Ljungdahl L. G., Dervartanian D. V., Watt G. D. Isolation and characterization of two rubredoxins from Clostridium thermoaceticum. Biochim Biophys Acta. 1980 Mar 7;590(1):24–33. doi: 10.1016/0005-2728(80)90143-7. [DOI] [PubMed] [Google Scholar]
- Yang S. S., Ljungdahl L. G., LeGall J. A four-iron, four-sulfide ferredoxin with high thermostability from Clostridium thermoaceticum. J Bacteriol. 1977 Jun;130(3):1084–1090. doi: 10.1128/jb.130.3.1084-1090.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Sone N., Hirata H., Kagawa Y. A highly stable adenosine triphosphatase from a thermophillie bacterium. Purification, properties, and reconstitution. J Biol Chem. 1975 Oct 10;250(19):7910–7916. [PubMed] [Google Scholar]



