Abstract
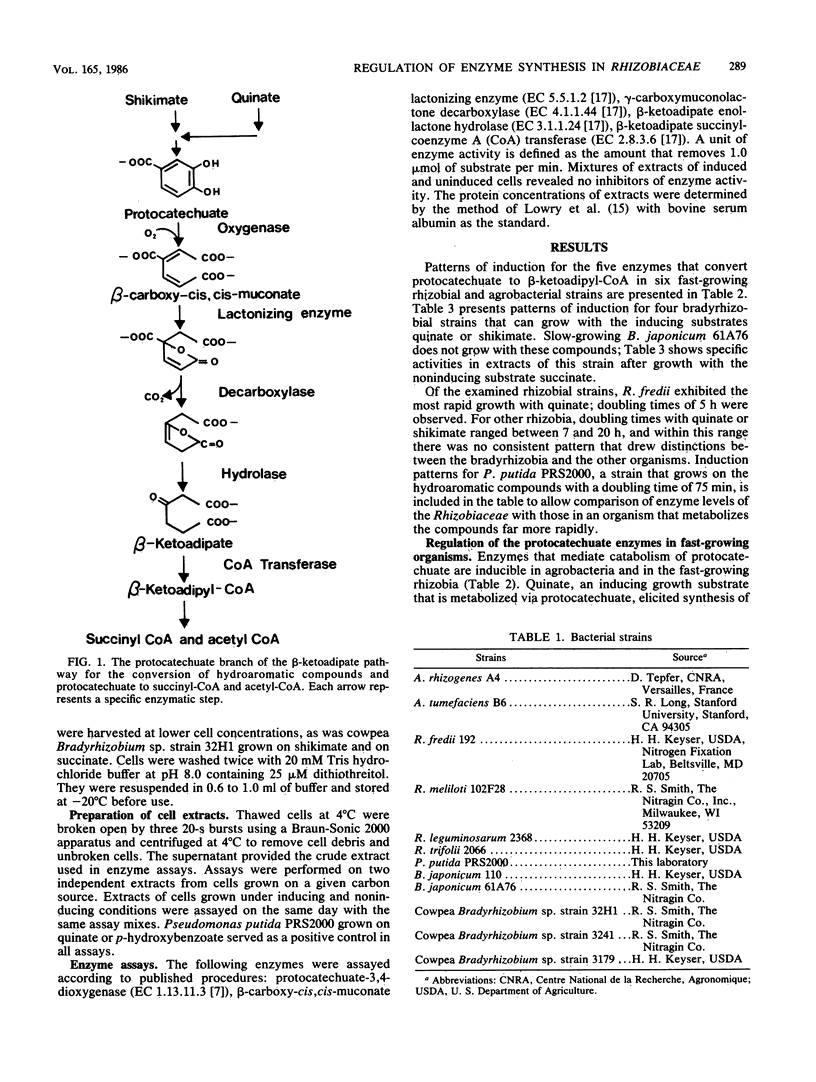
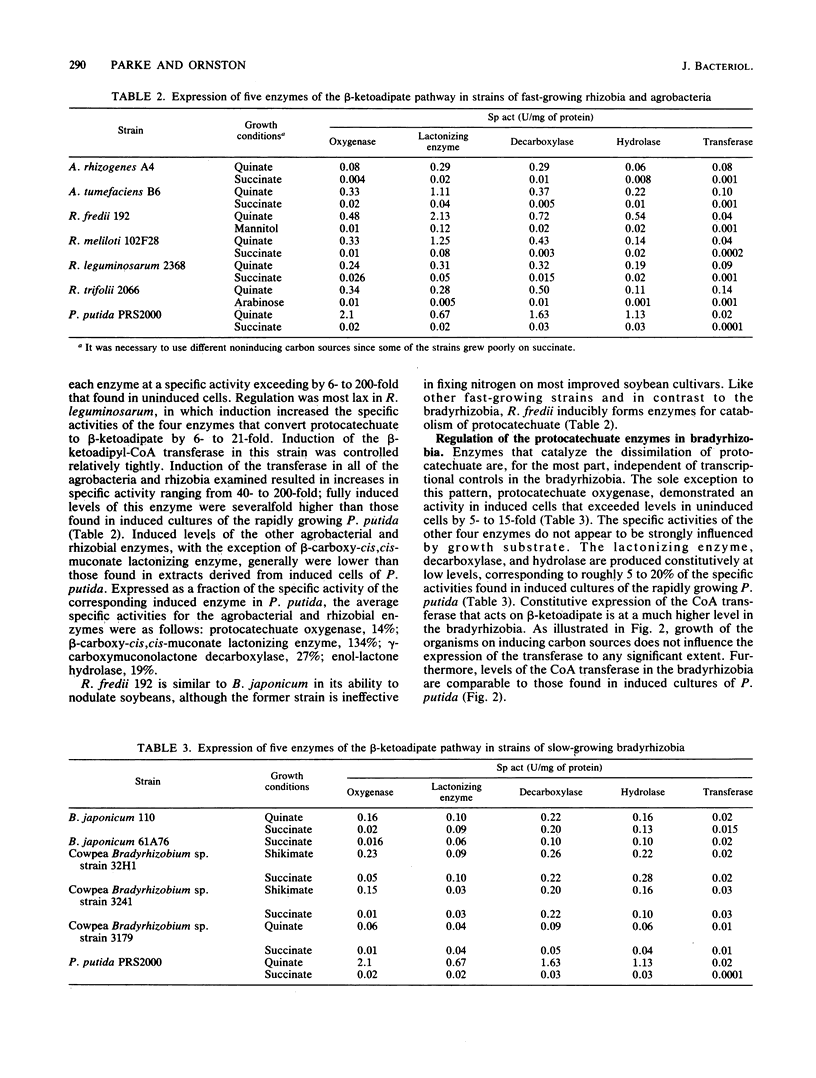
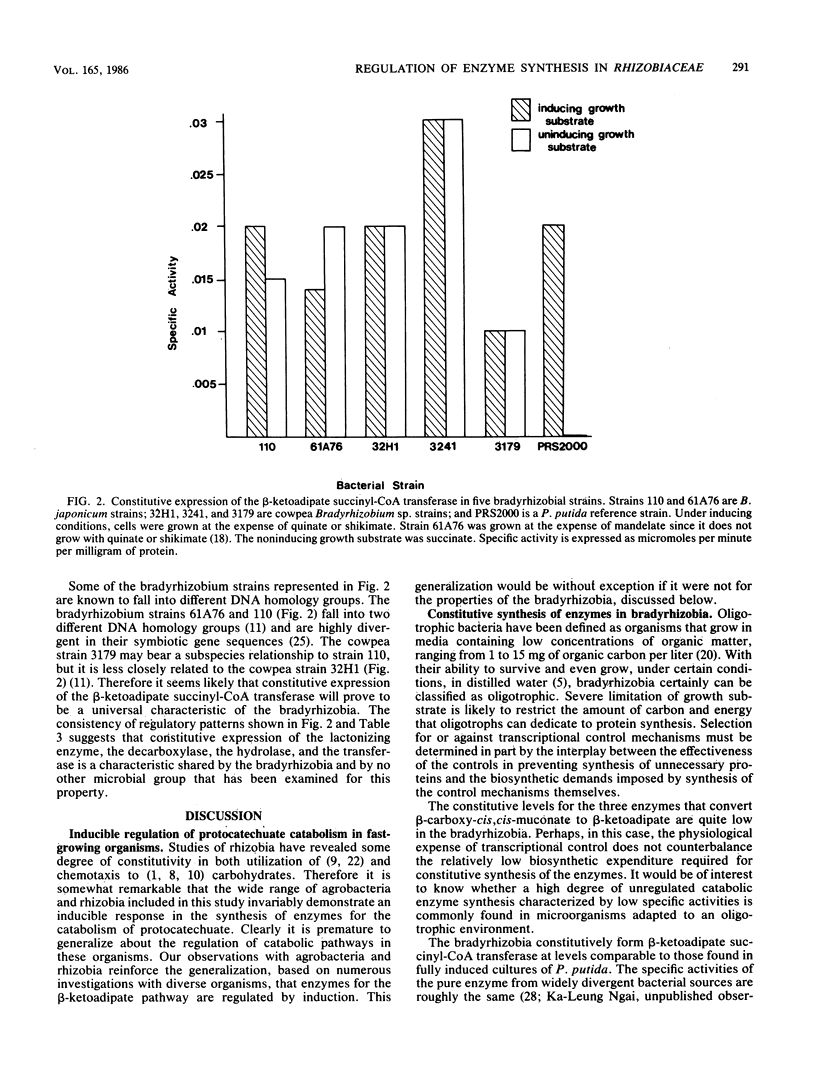
Protocatechuate is a universal growth substrate for members of the family Rhizobiaceae, and these bacteria utilize the aromatic compound via the beta-ketoadipate pathway. This report describes transcriptional controls exercised by different subgroups of the Rhizobiaceae over five enzymes that catalyze consecutive reactions in the pathway: protocatechuate oxygenase (EC 1.13.11.3), beta-carboxy-cis,cis-muconate lactonizing enzyme (EC 5.5.1.2), gamma-carboxymuconolactone decarboxylase (EC 4.1.1.44), beta-ketoadipate enol-lactone hydrolase (EC 3.1.1.24), and beta-ketoadipate succinyl-coenzyme A transferase (EC 2.8.3.6). All five enzymes were inducible in the fast-growing strains Agrobacterium rhizogenes, Agrobacterium tumefaciens, Rhizobium fredii, Rhizobium meliloti, Rhizobium leguminosarum, and Rhizobium trifolii. Specific activities in induced cells ranged from 5- to 100-fold greater than those found in uninduced cells. In contrast to the fast-growing strains and members of every other microbial taxon examined to date, the slow-growing Bradyrhizobium japonicum and cowpea Bradyrhizobium spp. constitutively expressed four of the five enzymes; protocatechuate oxygenase was the only inducible enzyme in this group. The slow-growing strains included different DNA homology groups, so it appears likely that constitutive expression of the four enzymes is a common trait in the bradyrhizobia. This property points to the importance of aromatic compounds and aromatic catabolites in the nutrition of these organisms.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cain R. B., Bilton R. F., Darrah J. A. The metabolism of aromatic acids by micro-organisms. Metabolic pathways in the fungi. Biochem J. 1968 Aug;108(5):797–828. doi: 10.1042/bj1080797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist D. K., Wyza R. E., Mills K. K., Bauer W. D., Evans W. R. Preservation of Rhizobium viability and symbiotic infectivity by suspension in water. Appl Environ Microbiol. 1984 May;47(5):895–900. doi: 10.1128/aem.47.5.895-900.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagley S. Catabolism of aromatic compounds by micro-organisms. Adv Microb Physiol. 1971;6(0):1–46. doi: 10.1016/s0065-2911(08)60066-1. [DOI] [PubMed] [Google Scholar]
- Durham D. R., Stirling L. A., Ornston L. N., Perry J. J. Intergeneric evolutionary homology revealed by the study of protocatechuate 3,4-dioxygenase from Azotobacter vinelandii. Biochemistry. 1980 Jan 8;19(1):149–155. doi: 10.1021/bi00542a023. [DOI] [PubMed] [Google Scholar]
- KILBY B. A. The formation of beta-ketoadipic acid by bacterial fission of aromatic rings. Biochem J. 1951 Oct;49(5):671–674. doi: 10.1042/bj0490671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser H. H., Bohlool B. B., Hu T. S., Weber D. F. Fast-growing rhizobia isolated from root nodules of soybean. Science. 1982 Mar 26;215(4540):1631–1632. doi: 10.1126/science.215.4540.1631. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Parke D., Ornston L. N. Constitutive synthesis of enzymes of the protocatechuate pathway and of the beta-ketoadipate uptake system in mutant strains of Pseudomonas putida. J Bacteriol. 1976 Apr;126(1):272–281. doi: 10.1128/jb.126.1.272-281.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parke D., Rivelli M., Ornston L. N. Chemotaxis to aromatic and hydroaromatic acids: comparison of Bradyrhizobium japonicum and Rhizobium trifolii. J Bacteriol. 1985 Aug;163(2):417–422. doi: 10.1128/jb.163.2.417-422.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SISTROM W. R., STANIER R. Y. The mechanism of formation of beta-ketoadipic acid by bacteria. J Biol Chem. 1954 Oct;210(2):821–836. [PubMed] [Google Scholar]
- Stanier R. Y., Ornston L. N. The beta-ketoadipate pathway. Adv Microb Physiol. 1973;9(0):89–151. [PubMed] [Google Scholar]
- Stanley J., Brown G. G., Verma D. P. Slow-growing Rhizobium japonicum comprises two highly divergent symbiotic types. J Bacteriol. 1985 Jul;163(1):148–154. doi: 10.1128/jb.163.1.148-154.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]



