Abstract
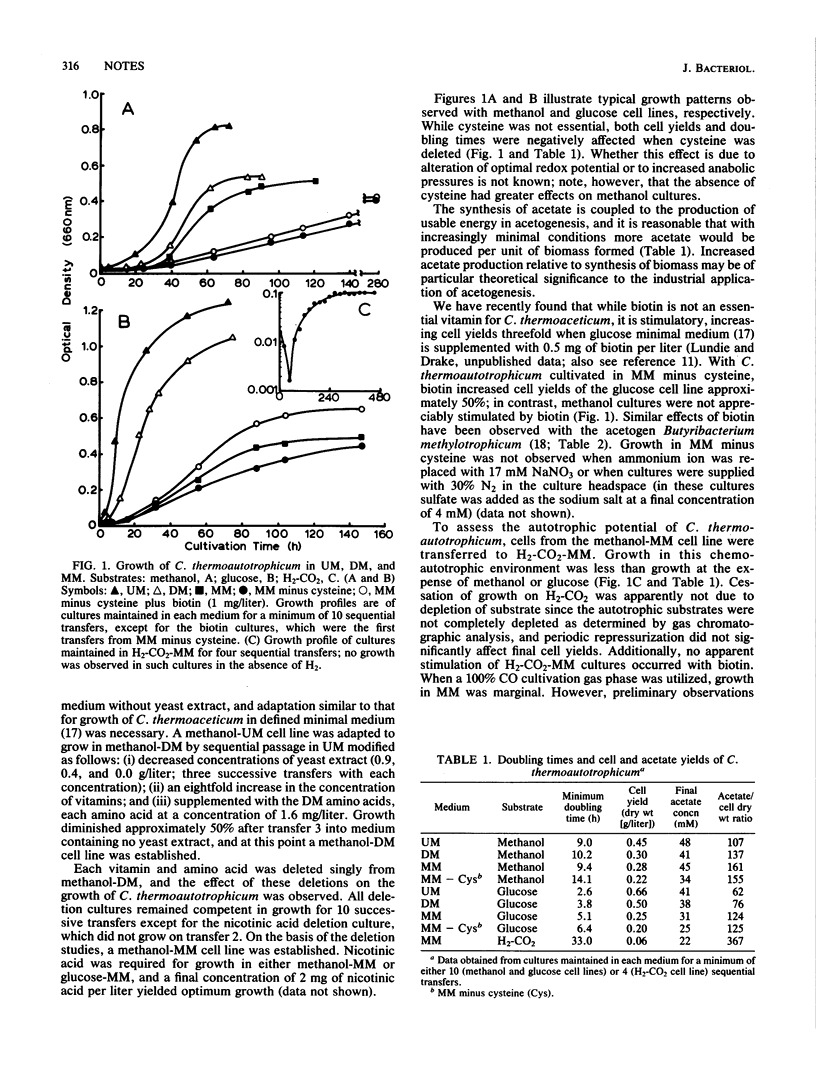
Clostridium thermoautotrophicum was adapted to minimal medium and cultivated at the expense of glucose, methanol, or H2-CO2. No supplemental amino acids were required for growth of the adapted strain, and nicotinic acid was the sole essential vitamin. Neither N2 nor nitrate could replace ammonium as the nitrogen source, and biotin was preferentially stimulatory for glucose cell lines. Growth in minimal medium yielded substantially higher acetate concentrations per unit of biomass formed than did growth in undefined medium.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamse A. D. New isolation of Clostridium aceticum (Wieringa). Antonie Van Leeuwenhoek. 1980;46(6):523–531. doi: 10.1007/BF00394009. [DOI] [PubMed] [Google Scholar]
- Adamse A. D., Velzeboer C. T. Features of a Clostridium, strain CV-AA1, an obligatory anaerobic bacterium producing acetic acid from methanol. Antonie Van Leeuwenhoek. 1982;48(4):305–313. doi: 10.1007/BF00418284. [DOI] [PubMed] [Google Scholar]
- Braun M., Mayer F., Gottschalk G. Clostridium aceticum (Wieringa), a microorganism producing acetic acid from molecular hydrogen and carbon dioxide. Arch Microbiol. 1981 Jan;128(3):288–293. doi: 10.1007/BF00422532. [DOI] [PubMed] [Google Scholar]
- Braun M., Schoberth S., Gottschalk G. Enumeration of bacteria forming acetate from H2 and CO2 in anaerobic habitats. Arch Microbiol. 1979 Mar 12;120(3):201–204. doi: 10.1007/BF00423066. [DOI] [PubMed] [Google Scholar]
- Clark J. E., Ragsdale S. W., Ljungdahl L. G., Wiegel J. Levels of enzymes involved in the synthesis of acetate from CO2 in Clostridium thermoautotrophicum. J Bacteriol. 1982 Jul;151(1):507–509. doi: 10.1128/jb.151.1.507-509.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer A. C., Young L. Y. A gram-negative anaerobic bacterium that utilizes o-methyl substituents of aromatic acids. Appl Environ Microbiol. 1985 May;49(5):1345–1347. doi: 10.1128/aem.49.5.1345-1347.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genthner B. R., Davis C. L., Bryant M. P. Features of rumen and sewage sludge strains of Eubacterium limosum, a methanol- and H2-CO2-utilizing species. Appl Environ Microbiol. 1981 Jul;42(1):12–19. doi: 10.1128/aem.42.1.12-19.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson J. L., Volcani B. E., Barker H. A. The Nutritional Requirements of Clostridium aceticum. J Bacteriol. 1948 Dec;56(6):781–782. [PMC free article] [PubMed] [Google Scholar]
- Kellum R., Drake H. L. Effects of cultivation gas phase on hydrogenase of the acetogen Clostridium thermoaceticum. J Bacteriol. 1984 Oct;160(1):466–469. doi: 10.1128/jb.160.1.466-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhardt U., Andreesen J. R. Some properties of formate dehydrogenase, accumulation and incorporation of 185W-tungsten into proteins of Clostridium formicoaceticum. Arch Microbiol. 1977 Dec 15;115(3):277–284. doi: 10.1007/BF00446453. [DOI] [PubMed] [Google Scholar]
- Lorowitz W. H., Bryant M. P. Peptostreptococcus productus strain that grows rapidly with CO as the energy source. Appl Environ Microbiol. 1984 May;47(5):961–964. doi: 10.1128/aem.47.5.961-964.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundie L. L., Jr, Drake H. L. Development of a minimally defined medium for the acetogen Clostridium thermoaceticum. J Bacteriol. 1984 Aug;159(2):700–703. doi: 10.1128/jb.159.2.700-703.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohwaki K., Hungate R. E. Hydrogen utilization by clostridia in sewage sludge. Appl Environ Microbiol. 1977 Jun;33(6):1270–1274. doi: 10.1128/aem.33.6.1270-1274.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolin E. A., Wolfe R. S., Wolin M. J. Viologen dye inhibition of methane formation by Methanobacillus omelianskii. J Bacteriol. 1964 May;87(5):993–998. doi: 10.1128/jb.87.5.993-998.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]



