Abstract
Insights have emerged concerning insulin function during development, from the finding that apoptosis during chicken embryo neurulation is prevented by prepancreatic (pro)insulin. While characterizing the molecules involved in this survival effect of insulin, we found insulin-dependent regulation of the molecular chaperone heat shock cognate 70 kDa (Hsc70), whose cloning in chicken is reported here. This chaperone, generally considered constitutively expressed, showed regulation of its mRNA and protein levels in unstressed embryos during early development. More important, Hsc70 levels were found to depend on endogenous (pro)insulin, as shown by using antisense oligodeoxynucleotides against (pro)insulin mRNA in cultured neurulating embryos. Further, in the cultured embryos, apoptosis affected mainly cells with the lowest level of Hsc70, as shown by simultaneous Hsc70 immunostaining and terminal deoxynucleotidyltransferase-mediated UTP nick end labeling. These results argue in favor of Hsc70 involvement, modulated by embryonic (pro)insulin, in the prevention of apoptosis during early development and suggest a role for a molecular chaperone in normal embryogenesis.
Programmed cell death, a process recognized increasingly in development (1), and the factors modulating survival/death of embryonic cells are characterized poorly in the critical periods of gastrulation and neurulation. Apoptosis, as well as other developmental processes, is likely to be regulated by a complex, changing combination of growth factors (2, 3). Some of these factors are well known for their actions in adult organisms, as is the case with the pancreatic hormone insulin. Preproinsulin mRNA is also expressed in the early prepancreatic embryo, even before insulin-like growth factor I (IGF-I), at least in chicken (4, 5) and Xenopus (6). The widespread distribution of preproinsulin mRNA during gastrulation and neurulation, as well as the similar broad distribution of the insulin receptor mRNA (5) and protein (7), potentially implicates (pro)insulin in the regulation of cellular events in early development. Indeed, we demonstrated previously that insulin is an autocrine/paracrine survival signal (5). Interference with embryonic (pro)insulin and insulin receptor expression using antisense oligodeoxynucleotides (ODNs) thus increased apoptosis, both in culture and in ovo (5), supporting the involvement of insulin signaling in the regulation of programmed cell death. In addition, exogenous insulin suppresses apoptosis of chicken embryo lens cells (8) and maintains the survival of migratory neural crest precursors (9), among other effects on neural cells (reviewed in ref. 10). Although another member of this family, IGF-I, is widely recognized as a survival factor for many cell types (10–12), and its possible mechanism of action through the pI3 kinase/Akt pathway is beginning to be elucidated (13, 14), the physiological survival effect of (pro)insulin has received less attention.
Molecular chaperones assist folding, translocation, and assembly of newly synthesized proteins (reviewed in refs. 15 and 16) and also protect the cell from heat shock and other physiological or environmental stresses, increasing cell survival after stress (17, 18). The specific mechanisms involved in the cell survival effect of some chaperones have nonetheless been approached only recently (19–21). How extracellular survival factors might modulate these chaperones and whether these molecules mediate the factors’ antiapoptotic function have not been elucidated.
We have reported recently that a putative chicken heat shock cognate (Hsc)70, a noninducible member of the heat shock 70-kDa protein family, is the antigen recognized by the monoclonal antibody PM1 (22, 23). The PM1-positive cells in the chicken embryonic retina in culture depend on exogenous insulin and IGF-1 (24). Because insulin prevents apoptosis and maintains the PM1/Hsc70-expressing cells, we have begun to explore a possible relationship between these phenomena that could prove to be part of the apoptosis prevention mechanism by embryonic (pro)insulin. We report here the isolation and characterization of the chicken hsc70 cDNA and its mRNA and protein expression from gastrulation to early organogenesis. As hypothesized, endogenous (pro)insulin maintained embryonic Hsc70 protein levels, and the apoptosis induced by growth factor deprivation, which is attenuated by insulin, was prevalent in cells with low Hsc70 levels.
EXPERIMENTAL PROCEDURES
Chicken Embryos and Treatments in Culture.
Fertilized White Leghorn eggs (Granja Rodríguez-Serrano, Salamanca, Spain) were incubated at 38.4°C, 60–90% relative humidity. The embryos were staged according to Hamburger and Hamilton (25). Embryos at 1.5 days of development (E1.5) were cultured in chemically defined medium as described (4, 26). Where indicated, embryos were treated with purified chicken insulin (Litron Lab, Rochester, NY), recombinant human insulin (a kind gift from Eli Lilly), or 12.5 nmol of the indicated ODN as described (see refs. 5 and 26 for extensive details and controls). All ODNs were synthesized as phosphorothioate derivatives by Oligos Etc. (Wilsonville, OR). Sequences were ASA, 5′-GAGAGCCATGATGAG-3′; ASB, 5′-GCTCGACATCCCGTC-3′; RAN, 5′-TAACGGTAACGGAGG-3′; SS, 5′-CTCATCATGGCTCTC-3′; ASIGF-I 5′-TTTTTCCATTGCTTC-3′). The inhibitors Ac-YVAD-CHO (for caspase 1) and Ac-DEVD-CHO (for caspase 3) or a control peptide inhibitor, Ac-LLR-CHO (leupeptin), were also used (Bachem). The inhibitors were added to the agarose under the embryo and to the medium at the indicated concentrations. After 5 h, a second dose was added to the medium.
Reverse Transcription PCR Cloning, cDNA Library Screening and Sequence Analysis.
Total RNA was extracted from the brain of embryos at 4.5 days of development (E4.5) by the RNAzol method (Biotecx Laboratories Inc., Houston). The RNA was reverse-transcribed using the First-Strand cDNA Synthesis Kit (Pharmacia), with the antisense primer 5′-GGIGGIGAIGA(C/T)TT(C/T)GA(C/T)AA-3′. The reverse transcription product was subjected to PCR amplification using 10 mM Tris, 50 mM KCl, 200 μM each dNTP, 1 mM MgCl2, 0.5 nM antisense primer, and 0.5 nM sense primer 5′-CCICCIACIA(A/G)IACIAT(A/G)TC(A/G)TG-3′; primers were derived from the known peptides sequence (23). The 309-bp amplified product was sequenced automatically by the dideoxynucleotide method (Perkin–Elmer) and cloned into a pGEM-T (Promega) vector. The sequence was compared by using the GCG software package and showed the highest identity with the human hsc70 gene. This fragment was labeled with digoxigenin-dUTP (Boehringer Mannheim) by random priming and was used as probe. An E1.5 chicken embryo cDNA library (kindly provided by R. M. Grainger, University of Virginia, Charlottesville) was screened by using standard methods. Of 13 positive plaques identified initially from 250,000 phages plated, 4 were subjected to three additional rounds of screening before purifying λ phage DNA with affinity columns (Wizard Lambda Preps, Promega). The four inserts were subcloned into the EcoRI site of pBluescript (Stratagene). Recombinant plasmids were subjected to restriction analysis and sequenced, resulting in a unique clone of 2120 bp.
Northern Blot Analysis.
Total RNA was extracted from whole embryos and 10 μg was electrophoresed, transferred, and crosslinked to filters by standard methods. The 309-bp hsc70 cDNA fragment used for the library screening and a 1.8-kb chicken hsp70 cDNA probe (a kind gift of R. I. Morimoto, Northwestern University, Evanston, IL) were used for hybridization of the same filters. Methylene blue staining of the membranes allowed control of loading.
Immunoblotting.
Crude embryonic extracts (solubilized at 25 mg of wet weight per ml of SDS-PAGE sample buffer) were fractionated and transferred to nitrocellulose membranes as described (22, 23). Blots were incubated with one of the following primary antibodies: mouse mAb PM1 (mouse ascites, 1/5,000 dilution), mouse mAb anti-Hsp70 (SPA-810, 1/1,000, StressGen Biotechnologies, Victoria, Canada), rat mAb anti-Hsc70 (SPA-815, 1/1,000, StressGen Biotechnologies, Victoria, Canada); mouse antiserum anti-Hsc70pep (1/3,000; generated against a synthetic polypeptide comprising amino acids 617–635 of the chicken Hsc70; see ref. 23 for details), or mouse mAb anti-β-tubulin (1/5,000, Sigma). Secondary antibodies were peroxidase-conjugated goat anti-mouse Ig (1/30,000, Jackson ImmunoResearch) or goat anti-rat Ig (1/6,000, Southern Biotechnology Associates). Incubation and washing steps were as described (22). Immunoblots were developed by using the ECL system (Amersham). Densitometric analysis was performed by using imagequant software (Molecular Dynamics).
Cell Dissociation and Nuclear Staining.
Cultured embryos were fixed in 4% (wt/vol) paraformaldehyde for 2 h and washed twice with PBS containing 3 mg/ml BSA. Embryos were then dissociated to single-cell suspension, deposited on a slide by using a cytospin, and stained as described (5, 27). Cells were stained and mounted with 4 μg/ml 4′,6-diamidino-2-phenylindole (Sigma), and 1 mg/ml o-phenylenediamine (Sigma) in glycerol:PBS (9:1). Pyknotic nuclei were observed under epifluorescence illumination in a Zeiss Axioscop.
Terminal Deoxynucleotidyltransferase-Mediated UTP Nick End Labeling (TUNEL) and Hsc70 Staining and Flow Cytometry.
Cultured embryos were processed as above. Aliquots of the cell suspension, typically 50,000 cells, were stained with rat mAb anti-Hsc70 (1.7 μg/ml) or with Igs as negative control, followed by biotinylated goat anti-mouse Ig (1/200 dilution, Amersham) and then with Cy2-streptavidin (1/200 dilution, Amersham). Fragmented DNA was stained by the TUNEL method in the same cell suspensions and was permeated with 0.6% (wt/vol) Triton X-100 in 2× SSPE. Samples were incubated 1 h at 37°C with 50 μl of a solution containing biotin-16-dUTP and terminal-deoxynucleotidyl-transferase, as indicated by the manufacturer (Boehringer Mannheim). The reaction was terminated by a 2-h incubation in 2× SSPE at 65°C. The end-labeling with biotin-16-dUTP was visualized with Cy3-streptavidin (1/100, Amersham). Flow cytometry data collection and multiparameter analysis were done in an EPICS XL Coulter Cytometer, as described (28).
RESULTS
Cloning of Chicken hsc70 cDNA.
The biochemical data indicating that the antigen recognized by mAb PM1 was the chaperone Hsc70 (23) were confirmed by the isolation of a full length 2,120-bp cDNA clone. The deduced amino acid sequence (Fig. 1) showed the highest homology with human Hsc70 (98% identity) among the nine members of the 70-kDa heat shock protein family cloned in humans and less with the other two members of the family known in chicken (86% with Hsp70 and 79% with Grp78).
Figure 1.
Predicted amino acid sequence of chicken Hsc70 (cHSC70), compared with chicken Hsp70 (cHSP70), Grp78 (cGRP78), and human Hsc70 (hHSC70). The amino acids conserved in all sequences are in black boxes, those conserved only in some of the sequences are in gray boxes. European Molecular Biology Laboratory nucleotide sequence database accession number for chicken Hsc70 is AJ004940.
Hsc70 Expression Is Regulated During Early Chicken Development.
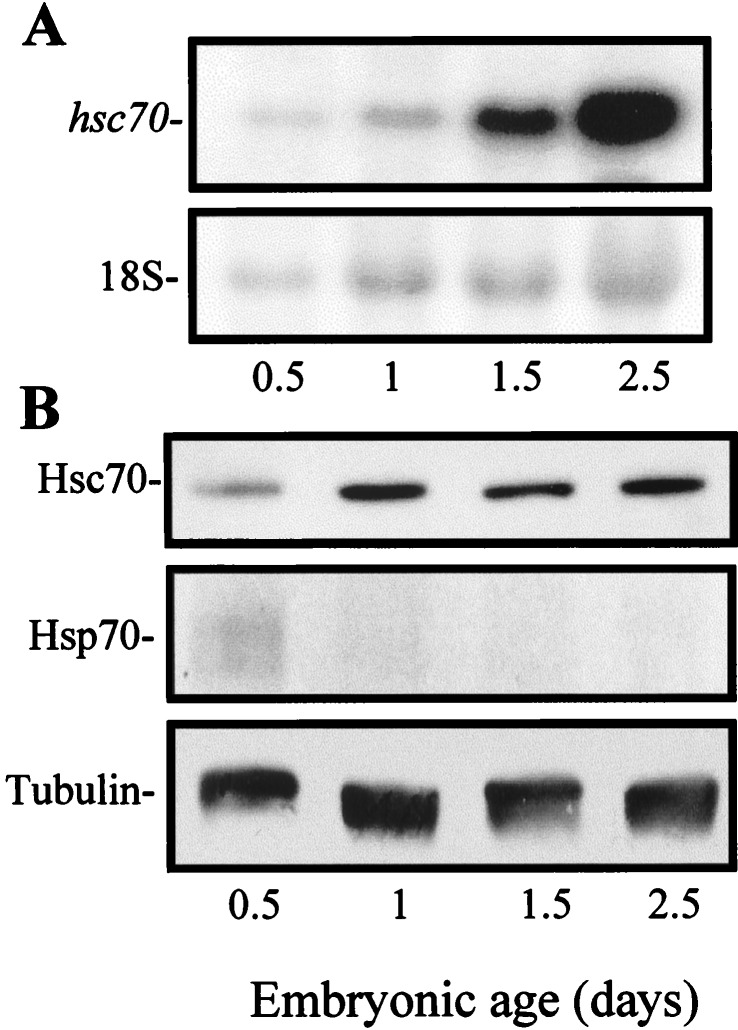
The chaperone Hsc70, generally considered constitutive, showed a developmentally regulated pattern of expression at both the mRNA and protein levels (Fig. 2). As early as gastrulation (E0.5), hsc70 mRNA was found in the embryo as a single 2.2-kb transcript, which increased 2.8-fold from gastrulation to early organogenesis (E2.5; Fig. 2A). In situ hybridization showed not a simple general increase in the hsc70 mRNA levels but a dynamic pattern with changing distribution as development proceeds (unpublished results). Rehybridization of the Northern blot with a chicken hsp70 probe gave no signal at any of these stages (data not shown). At the protein level (Fig. 2B), Hsc70 also revealed a developmentally regulated expression in parallel to that of its mRNA. Again, antigen distribution presented very elaborated patterns (unpublished results). Hsp70 was detected only at E0.5 and with much longer exposure time.
Figure 2.
Expression of Hsc70 mRNA and protein in early embryos. (A) Northern blot analysis of hsc70 mRNA in whole chicken embryos at the indicated ages. A single 2.2-kb transcript was revealed. Methylene blue staining of the 18S rRNA in the same blot (Lower) allowed for loading correction. The relative densitometric values (mean of three blots) after 18S correction were: 1 (E0.5), 1 (E1), 2 (E1.5), and 2.8 (E2.5). (B) Embryonic extracts of the indicated ages were subjected to SDS-PAGE and immunoblotting. Staining with antiserum anti-Hsc70pep revealed a 73-kDa band at all ages. In a parallel blot stained with mAb anti-Hsp70, only a faint band was observed at E0.5, despite the fact that the blot was exposed 8-fold longer than that stained for Hsc70. All the blots were restained for tubulin to allow for loading correction. The relative Hsc70 densitometric values (mean of four blots) after tubulin correction were: 1 (E0.5), 1 (E1), 1.3 (E1.5), and 1.8 (E2.5).
Embryonic Apoptosis Attenuated by Insulin Involves Caspases.
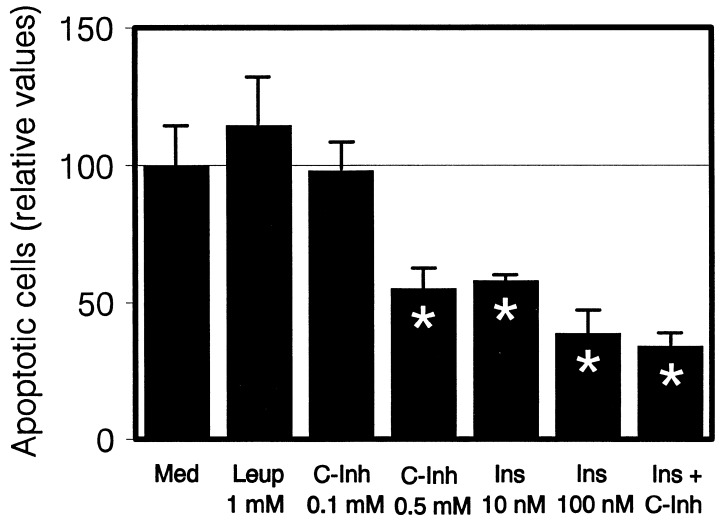
E1.5 embryos in growth factor-deprived culture undergo apoptosis, which is attenuated by insulin as the only exogenous growth factor (5). To approach the mechanisms of this apoptotic process and of insulin action, we used peptide inhibitors of the caspases, the main executors of apoptotic death (29). Apoptosis induced in neurulating embryos by growth factor deprivation was rescued up to 50% by the addition of two caspase inhibitors (Fig. 3), demonstrating the involvement of caspases in this embryonic cell death. Exogenous insulin, as described, also attenuated apoptotic cell death in a dose-dependent manner, with a maximal effect of 70% rescue at 10−7 M. The simultaneous addition of both caspase inhibitors and insulin at their respective maximal effective concentrations did not produce additive effects, suggesting that insulin prevented the death program executed by caspases in the early embryo. When caspase activity was measured, however, there was still some activity inhibitable by caspase inhibitors in the presence of insulin (data not shown).
Figure 3.
Protection from apoptosis by caspase inhibitors and insulin. E1.5 embryos were cultured in basal medium alone (Med) or with the indicated concentrations of leupeptin (Leup), caspase 1 and 3 inhibitors (C-Inh), chicken insulin (Ins), or 100 nM insulin and 0.5 mM each caspase inhibitors together. After 10 h of culture, apoptosis was quantified in dissociated cells. Six hundred cells were counted per experimental point. The values represent the mean ± SEM of three to five cultured embryos in each of two to eight different experiments and are expressed relative to the embryos cultured in medium alone. ∗, P < 0.001 with respect to the medium alone.
Hsc70 Level Is Modulated by Embryonic (Pro)insulin.
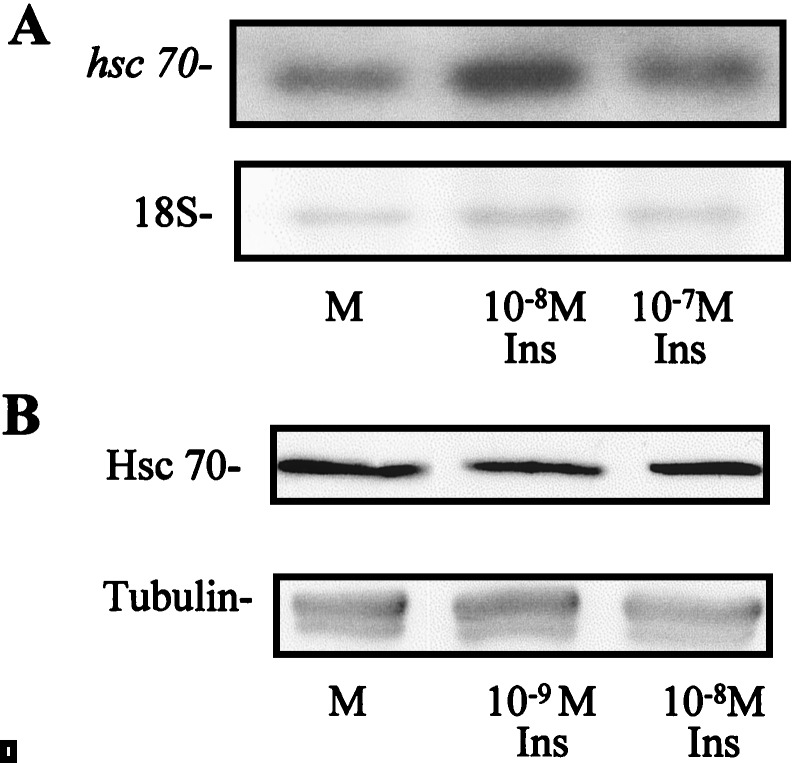
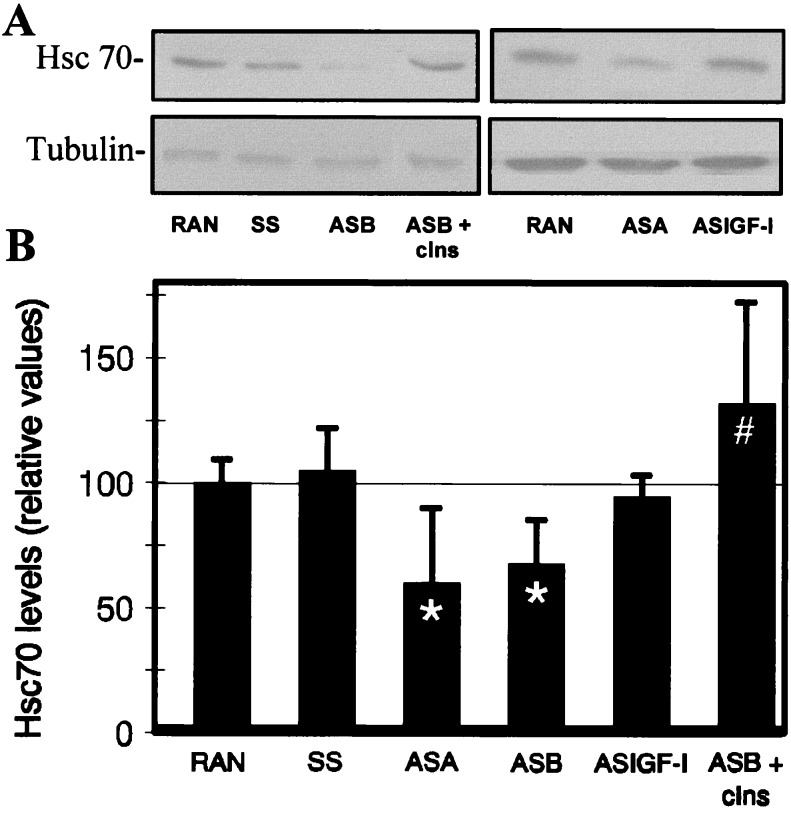
We tested whether (pro)insulin was able to maintain higher levels of Hsc70 in addition to preventing apoptotic cell death. Exogenous insulin added to cultured embryos, however, increased Hsc70 mRNA and protein only modestly (Fig. 4). This low stimulation, borderline significant, could be in part secondary to the upregulation of the embryonic endogenous (pro)insulin mRNA expression observed in the cultured embryos (4), which attempts to overcome growth-factor deprivation. Therefore, we inhibited the biosynthesis of embryonic (pro)insulin by antisense ODNs, a strategy used successfully in this system (5, 26). Antisense ODNs complementary to the chicken (pro)insulin mRNA were applied to E1.5 embryos in culture and Hsc70 was analyzed by immunoblotting using mAb PM1 and antiserum anti-Hsc70pep (Fig. 5). As hypothesized, endogenous (pro)insulin was involved in maintaining Hsc70 levels. The antisense ODNs (ASA and ASB) reduced the level of Hsc70 by 40%, a significant decrease when compared with control ODNs (RAN and SS). Antisense ODN complementary to chicken IGF-I (ASIGF-I) had no effect on Hsc70 levels, supporting previous data showing that IGF-I signaling through its receptor is less relevant than (pro)insulin signaling in the neurulating embryo (5). Consequently, 10−7 M chicken insulin added to the culture medium together with ASB recovered Hsc70 level, confirming the specificity of the (pro)insulin antisense ODN effect, and further increased Hsc70 levels by 32% with respect to control embryos. These results are fully concordant with the reported effects of (pro)insulin antisense ODN treatment on apoptosis. ASA and ASB increased apoptosis by 40% in cultured embryos (5). Additionally, in parallel with Hsc70 modulation, β-actin, which is transcriptionally regulated by exogenous insulin in proliferating cells (30), increased in the presence of insulin and decreased with ASB (data not shown), whereas β-tubulin showed no significant changes on similar treatments (Fig. 5).
Figure 4.
Exogenous insulin increases Hsc70 mRNA and protein levels in cultured embryos. (A) Northern blot analysis of hsc70 mRNA in whole chicken embryos cultured in basal medium (M) or in the presence of insulin (Ins) for 12 h. The single 2.2-kb transcript was quantified relative to methylene blue-stained 18S rRNA (Lower). The relative densitometric values (mean of two blots) were 1 (M), 1.3 (10−8 M insulin) and 1.2 (10−7 M insulin). (B) Extracts of embryos cultured in basal medium or in the presence of insulin for 12 h were subjected to SDS-PAGE and immunoblotting. The staining with antiserum anti-Hsc70pep revealed a 73-kDa band that was quantified relative to tubulin (Lower). The relative Hsc70 densitometric values (mean of four blots) were 1 (M), 1.16 (10−9 M insulin), 1.25 (10−8 insulin).
Figure 5.
Hsc70 levels decrease after treatment with (pro)insulin antisense oligonucleotides. (A) Two representative blots, illustrating the different treatments, with extracts from cultured E1.5 embryos treated with the indicated insulin antisense (ASA, ASB), IGF-I antisense (ASIGF-I), or control (RAN, SS) ODNs, and 10−7 M chicken insulin where indicated. After 10 h in culture, the extracts from individual embryos were subjected to SDS-PAGE and immunoblotting with mAb PM1 or with antiserum anti-Hsc70pep. Blots were restained for tubulin for loading correction and quantified by densitometry. (B) Quantitation of ODN effect on Hsc70 levels. Values represent the mean ± SEM of two to three replicate immunoblots from each of two to three embryos treated per point in each of two to three independent experiments and are expressed relative to control RAN ODN treated embryos in the same experiment. ∗, P < 0.025 relative to the RAN treatment; #, P < 0.025, relative to the ASB treatment.
Prevalence of Apoptotic Death in Embryonic Cells with Low Hsc70 Content.
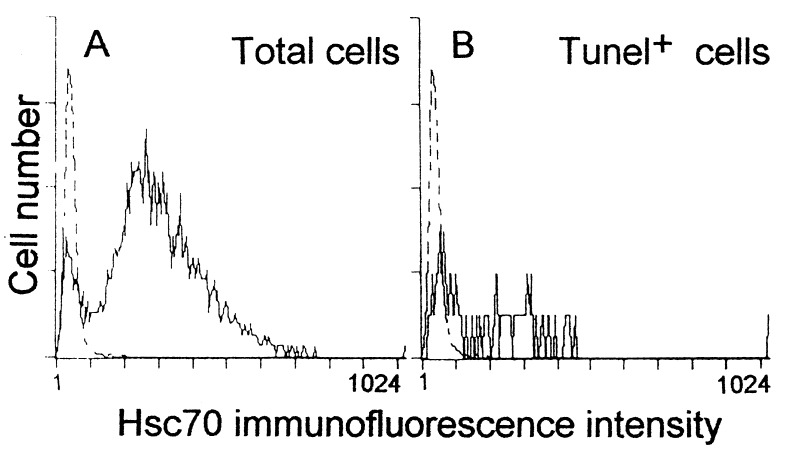
Because embryonic (pro)insulin prevented apoptosis and maintained overall embryo Hsc70, we sought a relationship between cell survival and Hsc70 expression. A highly sensitive detection and quantitation by flow cytometry demonstrated that there was graded Hsc70 expression in the dispersed E1.5 embryo cells, with up to 85% of the cells containing levels measurable above background (Fig. 6A). This graded expression is also reflected in immunohistochemistry (unpublished results). In embryos cultured for a short time (6 h), apoptotic cell death could be detected by TUNEL staining simultaneously with Hsc70 staining. The TUNEL-stained cells presented low Hsc70 immunofluorescence signal (Fig. 6B); conversely, the proportion of dead cells was higher among the cells with low Hsc70 levels (data not shown).
Figure 6.
Flow cytometric analysis of Hsc70- and TUNEL-stained cells from E1.5 embryos cultured for 6 h in basal medium. Double TUNEL and Hsc70 immunostaining was performed in dissociated cells. (A) One representative Hsc70 staining (continuous line) of total cells (of 15 embryos in five independent experiments). Nonimmune Ig was used as a control (dotted line). (B) Hsc70 immunofluorescence levels were also analyzed in the TUNEL-stained cells. Note that 45% of apoptotic cells coincided with the area considered negative for Hsc70 (dotted line) and 90% of the TUNEL-stained cells showed an Hsc70 immunofluorescence signal below the mean signal of the total cell population. The profiles were similar for embryos cultured with insulin, but the proportion of apoptotic cells was lower.
DISCUSSION
This study supports the involvement of embryonic (pro)insulin in early developmental processes, in particular in the regulation of cell survival/death, through attenuation of caspase-executed cell death, possibly by maintaining Hsc70 levels. In addition, the developmental and growth factor-dependent regulation of the generally considered constitutive chaperone Hsc70 in unstressed embryos has been demonstrated.
Cloning of chicken Hsc70 has confirmed further the biochemical nature of the antigen recognized by mAb PM1 (22, 23). As expected, chicken Hsc70 presented higher homology with Hsc70s cloned in vertebrates than with other members of the 70-kDa heat shock protein family, such as Hsp70, a heat-inducible chaperone already described in chicken (31). Hsc70 mRNA and protein were relatively abundant in early unstressed chicken embryos, whereas Hsp70 was demonstrable only at very low protein levels in the E0.5 embryo. Hsc70 presented not only a distinctive developmental pattern but also graded expression in the neurulating embryo, as visualized by flow cytometry (Fig. 6) and by in situ hybridization and immunofluorescence (unpublished results). Hsc70 cellular levels are modulated also in the developing neuroretina, where Hsc70 is restricted to proliferating neuroepithelial cell subpopulations and to ganglion cell neurons (22, 23). The precise regulation and the extreme differences found in Hsc70 cell content in developmental systems are difficult to relate directly to a generalized role such as a molecular chaperone assisting protein folding and preventing misfolding, both in normal and stressed conditions (15, 16).
A role for the various chaperone families in the development of different species is indeed suspected, based on observations of their expression in embryos, but their specific function in developmental processes is undefined. The initial recognition of Hsc70 proteins distinct from Hsp70 in Drosophila was already suggestive of their developmental importance, because transcript levels for Hsc1, Hsc2, and Hsc4 varied from embryos to larvae to adult flies (32). In mouse, the synthesis of cognate and inducible members of the chaperone/heat shock 70-kDa protein family was demonstrated in the two-cell embryo at the time of onset of zygotic genome activation (33). Hsp70 expression, which occurs here in the absence of experimental stress, is repressed subsequently and does not exhibit a stress response until the blastocyst stage. In contrast, Hsc70 synthesis has been reported in all developmental stages of mouse embryogenesis from E8.5 (neurulation) and in all tissues examined, with no significant variations (34, 35), a pattern distinct from that described here for early chicken embryogenesis. Other authors, however, have found that Hsc70 expression occurs in early mouse development at the blastula stage and is down-regulated toward the end of embryogenesis in almost all tissues except for nervous tissue, which retains high expression (36, 37). Similarly, Xenopus Hsc70.I is expressed from cleavage to tadpole stages, showing a significant increase after the blastula stage that correlates with activation of the embryonic genome at the midblastula transition (38). In addition, Hsp70 is constitutively expressed during amphibian oogenesis, increasing in oocytes from stage II to stage VI under physiological conditions (39–41). In the chicken embryo, Hsp70 expression has been described in the lens at E6, E14, and E19, with increasing levels that appeared associated with differentiated fiber cell formation (42). In contrast, in maturing erythrocytes, Hsp70 level decreased from E3 to E8 (43). In some of the above studies, however, the results may have been misinterpreted, because of the lack of specificity of the antibodies which could crossreact with Hsp70, Hsc70 and, possibly, with other members of the family. A direct developmental function for one member of the vertebrate 70-kDa chaperone protein family members was described recently in mouse. Hsp70–2 is a spermatocyte-specific chaperone, whose disruption provokes a lack of postmeiotic spermatids and mature sperm and, as a consequence, male infertility. The failure in meiosis in Hsp70–2 null mutant animals was coincident with a dramatic increase in spermatocyte apoptosis (44).
Some additional clues to the developmental roles of chaperones can be derived from our observations here of Hsc70 modulation by embryonic (pro)insulin in parallel to the prevention of apoptosis. An antisense ODN treatment that decreases embryonic (pro)insulin biosynthesis and induces apoptosis (5) also decreases the Hsc70 levels. Our data suggest that cells with low Hsc70 levels are more vulnerable to apoptosis induced by growth factor deprivation. Although it has been difficult to evaluate the changing Hsc70 levels during the process of cell death induced in culture, possibly because of the progressive loss of the cytosolic proteins, we labeled both DNA fragmentation by TUNEL and Hsc70 cell content at short culture times. This approach was, unfortunately, insufficiently discriminatory to determine which event occurred first, loss of Hsc70 or initiation of apoptotic cell death. Published evidence favors the interpretation of increased sensitivity to apoptosis by loss of Hsc70 (19–21). Overexpression of Hsp72 in neurons using viral vectors, in turn, reduced the number of neurons that died after stroke (45). The inhibition mechanism of stress-induced apoptosis by Hsp70/Hsc70 has been recently reported to reside at various levels, including inhibition of the activity of the stress-activated protein kinase SAPK/JNK, as well as prevention of caspase 3 processing (20). In addition, the antiapoptotic protein BAG-1 modulates the chaperone activity of both Hsc70 and Hsp70 (19, 21). Other points of chaperone interaction with death/survival pathways have implicated the tumor suppressor protein p53. In ras-transformed cell lines, Hsc70, and not Hsp70, is the chaperone that forms a stable association with p53 (46). In addition to their chaperone function, these proteins may have other roles in signal transduction, for instance by means of association with steroid receptors (47).
Further work will be needed to establish a direct link between insulin, Hsc70 expression, and cell survival. We know that (pro)insulin prevents apoptosis in the embryo acting through its own receptor, but we do not know whether Hsc70 levels are modified by insulin receptor activity. Indeed, insulin activation of the pathways leading to increased transcription through the SRE and the AP-1 sites of several genes is well established (reviewed in ref. 48). There is a 7-bp sequence (TGAGTGC) located at position −198 of the human hsc70 promoter (49), relatively similar to the TRE element (TGCGTCA), which acts as the AP-1 complex-binding site mediating insulin effect on the collagenase promoter (50). Work with IGF-I implicates the phosphatidylinositol-kinase pathway and the phosphorylation of BAD induced by Akt as the main apoptosis protective mechanism (13, 14); this pathway is also triggered by insulin receptor activation (reviewed in ref. 51). Regulation of embryonic chaperone/heat shock proteins by extracellular signals has not been addressed in detail. In a hepatoma cell line, insulin induced hsp70 mRNA, whereas epidermal growth factor, platelet-derived growth factor, glucocorticoids, and other factors did not (52). During amphibian metamorphosis, several Hsp40, Hsp60, Hsp90, and Grp78 homologues were found to be regulated by T3 (53). During labor in mammals, another “physiological stress,” estradiol, increases Hsp70 and Hsp90 in the myometrium (54). Early work in cell lines found that Hsp70 was induced by serum (55), and growth factors were generally presented as inducers of stress proteins (56). In the early chicken embryo, it is possible that other factors of the insulin family cooperate, maintaining survival pathways and chaperone expression. During neurulation, IGF-I is not expressed (4, 5), but IGF-II mRNA and protein are present (J.S., unpublished observations). As far as the protein form, (pro)insulin or insulin, actually present in the prepancreatic embryo, we hypothesize that it is unprocessed (pro)insulin, rapidly secreted through a constitutive pathway. Later in development, we have evidence that in neuroretina and liver, in contrast with pancreas, (pro)insulin is secreted largely unprocessed (unpublished observation).
The modulation of Hsc70 by embryonic insulin and its decrease in apoptotic cells reported here provide insight into the molecules involved in the control of cell survival/death by growth factors and encourages further analysis of the biochemical and cellular specificity of chaperone function during early vertebrate development.
Acknowledgments
We thank P. Aller for critical reading of the manuscript and E. Martínez and V. Quesada for technical assistance. This study was funded by Grants PB94-0052 to F.d.P. and PM96-0003 to E.J.d.l.R. from the Dirección General de Investigación y Desarrollo (Spain). Fellowship to E.V.-N. was awarded by the Comunidad Autónoma de Madrid, and fellowships to A.V.M., J.S., and E.R. were awarded by the Ministerio de Educación y Cultura (Spain).
ABBREVIATIONS
- Hsc70
heat shock cognate, 70 kDa
- IGF
insulin-like growth factor
- ODN
oligodeoxynucleotide
- En
embryonic day n
- TUNEL
terminal deoxynucleotidyltransferase-mediated UTP nick end labeling
Footnotes
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession no. AJ004940).
References
- 1.Weil M, Jacobson M D, Raff M C. Curr Biol. 1997;7:281–284. doi: 10.1016/s0960-9822(06)00125-4. [DOI] [PubMed] [Google Scholar]
- 2.Heath J K, Valancius-Mangel V. Curr Opin Cell Biol. 1991;3:935–938. doi: 10.1016/0955-0674(91)90110-k. [DOI] [PubMed] [Google Scholar]
- 3.Harris W A. Curr Opin Genet Dev. 1997;7:651–658. doi: 10.1016/s0959-437x(97)80013-5. [DOI] [PubMed] [Google Scholar]
- 4.Pérez-Villamil B, de la Rosa E J, Morales A V, de Pablo F. Endocrinology. 1994;135:2342–2350. doi: 10.1210/endo.135.6.7988416. [DOI] [PubMed] [Google Scholar]
- 5.Morales A V, Serna J, Alarcón C, de la Rosa E J, de Pablo F. Endocrinology. 1997;138:3967–3975. doi: 10.1210/endo.138.9.5396. [DOI] [PubMed] [Google Scholar]
- 6.Parfetti R, Scott L A, Shuldiner A R. Endocrinology. 1994;135:2037–2044. doi: 10.1210/endo.135.5.7956926. [DOI] [PubMed] [Google Scholar]
- 7.Girbau M, Bassas L, Alemany J, de Pablo F. Proc Natl Acad Sci USA. 1989;86:5868–5872. doi: 10.1073/pnas.86.15.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rampalli A M, Zelenka P S. Cell Growth Differ. 1995;6:945–953. [PubMed] [Google Scholar]
- 9.Nataf V, Monier S. Dev Brain Res. 1992;69:59–66. doi: 10.1016/0165-3806(92)90122-d. [DOI] [PubMed] [Google Scholar]
- 10.de Pablo F, de la Rosa E J. Trends Neurosci. 1995;18:143–150. doi: 10.1016/0166-2236(95)93892-2. [DOI] [PubMed] [Google Scholar]
- 11.Barres B A, Schmid R, Sendnter M, Raff M C. Development (Cambridge, UK) 1993;118:283–295. doi: 10.1242/dev.118.1.283. [DOI] [PubMed] [Google Scholar]
- 12.Harrington E A, Bennett M R, Fanidi A, Evan G I. EMBO J. 1994;13:3286–3295. doi: 10.1002/j.1460-2075.1994.tb06630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 14.Párrizas M, Saltier A R, LeRoith D. J Biol Chem. 1997;272:154–161. doi: 10.1074/jbc.272.1.154. [DOI] [PubMed] [Google Scholar]
- 15.Hartl F U. Nature (London) 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 16.Rassow J, Von Ahsen O, Bömer U, Pfanner N. Trends Cell Biol. 1997;7:129–133. doi: 10.1016/S0962-8924(96)10056-8. [DOI] [PubMed] [Google Scholar]
- 17.Li G C, Werb Z. Proc Natl Acad Sci USA. 1982;79:3218–3222. doi: 10.1073/pnas.79.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parsell D A, Lindquist S. Annu Rev Genet. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- 19.Höhfeld J, Jentsch S. EMBO J. 1997;16:6209–6216. doi: 10.1093/emboj/16.20.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mosser D D, Caron A W, Bourget L, Denis-Larose C, Massie B. Mol Cell Biol. 1997;17:5317–5327. doi: 10.1128/mcb.17.9.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takayama S, Bimston D N, Matsuzawa S-I, Freeman B C, Aime-Sempe C, Xie Z, Morimoto R I, Reed J C. EMBO J. 1997;16:4887–4896. doi: 10.1093/emboj/16.16.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernández-Sánchez C, Frade J M, de la Rosa E J. Eur J Neurosci. 1994;6:105–114. doi: 10.1111/j.1460-9568.1994.tb00252.x. [DOI] [PubMed] [Google Scholar]
- 23.Morales, A. V., Hadjiargyrou, M., Díaz, B., Hernández-Sánchez, C., de Pablo, F. & de la Rosa, E. J. (1998) Eur. J. Neurosci., in press. [DOI] [PubMed]
- 24.de la Rosa E J, Bondy C A, Hernández-Sánchez C, Wu X, Zhou J, López-Carranza A, Scavo L M, de Pablo F. Eur J Neurosci. 1994;6:1801–1810. doi: 10.1111/j.1460-9568.1994.tb00573.x. [DOI] [PubMed] [Google Scholar]
- 25.Hamburger V, Hamilton H L. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- 26.Morales A V, de Pablo F. Curr Top Dev Biol. 1998;36:37–49. doi: 10.1016/s0070-2153(08)60494-9. [DOI] [PubMed] [Google Scholar]
- 27.de la Rosa E J, Díaz B, de Pablo F. Curr Top Dev Biol. 1998;36:133–144. doi: 10.1016/s0070-2153(08)60499-8. [DOI] [PubMed] [Google Scholar]
- 28.Serna J, Pimentel B, de la Rosa E J. Curr Top Dev Biol. 1998;36:211–222. doi: 10.1016/s0070-2153(08)60504-9. [DOI] [PubMed] [Google Scholar]
- 29.Vaux D L, Strasser A. Proc Natl Acad Sci USA. 1996;93:2239–2244. doi: 10.1073/pnas.93.6.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Messina J L. Mol Endocrinol. 1992;6:112–119. doi: 10.1210/mend.6.1.1738364. [DOI] [PubMed] [Google Scholar]
- 31.Morimoto R I, Hunt C, Huang S Y, Berg K L, Banerji S S. J Biol Chem. 1986;261:12692–12699. [PubMed] [Google Scholar]
- 32.Craig E A, Ingolia T D, Monseau L J. Dev Biol. 1983;99:418–426. doi: 10.1016/0012-1606(83)90291-9. [DOI] [PubMed] [Google Scholar]
- 33.Bensaüde O, Babinet C, Morange M, Jacob F. Nature (London) 1983;305:331–333. doi: 10.1038/305331a0. [DOI] [PubMed] [Google Scholar]
- 34.Kothary R, Perry M D, Moran L A, Rossant J. Dev Biol. 1987;121:342–348. doi: 10.1016/0012-1606(87)90170-9. [DOI] [PubMed] [Google Scholar]
- 35.Manejwala F M, Logan C Y, Schultz R M. Dev Biol. 1991;144:301–308. doi: 10.1016/0012-1606(91)90423-z. [DOI] [PubMed] [Google Scholar]
- 36.Giebel L B, Dworniczak B P, Bautz E K. Dev Biol. 1988;125:200–207. doi: 10.1016/0012-1606(88)90073-5. [DOI] [PubMed] [Google Scholar]
- 37.Rallu M, Loones M, Lallemand Y, Morimoto R, Morange M, Mezger V. Proc Natl Acad Sci USA. 1997;94:2392–2397. doi: 10.1073/pnas.94.6.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ali A, Salter Cid L, Flajnik M F, Heikkila J J. Comp Biochem Physiol. 1996;113:681–687. doi: 10.1016/0305-0491(95)02081-0. [DOI] [PubMed] [Google Scholar]
- 39.Horrell A, Shuttleworth J, Colman A. Genes Dev. 1987;1:433–444. doi: 10.1101/gad.1.5.433. [DOI] [PubMed] [Google Scholar]
- 40.Billoud B, Rodríguez Martín M L, Berard L, Moreau N, Angelier N. Development. 1993;119:921–932. doi: 10.1242/dev.119.3.921. [DOI] [PubMed] [Google Scholar]
- 41.Herberts C, Moreau N, Angelier N. Int J Dev Biol. 1993;37:397–406. [PubMed] [Google Scholar]
- 42.Dash A, Chung S, Zelenka P S. Exp Eye Res. 1994;58:381–387. doi: 10.1006/exer.1994.1030. [DOI] [PubMed] [Google Scholar]
- 43.Banerji S S, Laing K, Morimoto R I. Genes Dev. 1987;1:946–953. doi: 10.1101/gad.1.9.946. [DOI] [PubMed] [Google Scholar]
- 44.Dix D J, Allen J W, Collins B W, Mori C, Nakamura N, Poorman-Allen P, Goulding E H, Eddy E M. Proc Natl Acad Sci USA. 1996;93:3264–3268. doi: 10.1073/pnas.93.8.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wickelgren I. Science. 1997;278:1405. doi: 10.1126/science.278.5342.1405. [DOI] [PubMed] [Google Scholar]
- 46.Hinds P W, Finlay C A, Frey A B, Levine A J. Mol Cell Biol. 1987;7:2863–2869. doi: 10.1128/mcb.7.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao N, DeFranco D B. Mol Endocrinol. 1997;11:1365–1374. doi: 10.1210/mend.11.9.9976. [DOI] [PubMed] [Google Scholar]
- 48.O’Brien R M, Granner D K. Physiol Rev. 1996;76:1109–1161. doi: 10.1152/physrev.1996.76.4.1109. [DOI] [PubMed] [Google Scholar]
- 49.Dworniczak B, Mirault M-E. Nucleic Acids Res. 1987;15:5181–5196. doi: 10.1093/nar/15.13.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tavaré J M, Rutter G A, Griffiths M R, Dobson S P, Gray H. Biochem Soc Trans. 1996;24:378–384. doi: 10.1042/bst0240378. [DOI] [PubMed] [Google Scholar]
- 51.Marte B M, Downward J. Trends Biochem Sci. 1997;22:355–358. doi: 10.1016/s0968-0004(97)01097-9. [DOI] [PubMed] [Google Scholar]
- 52.Ting L-P, Tu C-L, Chon C-K. J Biol Chem. 1989;264:3404–3408. [PubMed] [Google Scholar]
- 53.Buckbinder L, Brown D D. J Biol Chem. 1992;267:25786–25791. [PubMed] [Google Scholar]
- 54.Wu W X, Derks J B, Zhang Q, Nathanielsz P W. Endocrinology. 1996;137:5685–5693. doi: 10.1210/endo.137.12.8940400. [DOI] [PubMed] [Google Scholar]
- 55.Wu B J, Morimoto R I. Proc Natl Acad Sci USA. 1985;82:6070–6074. doi: 10.1073/pnas.82.18.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morimoto R I, Sarge K D, Abravaya K. J Biol Chem. 1992;267:21987–21990. [PubMed] [Google Scholar]