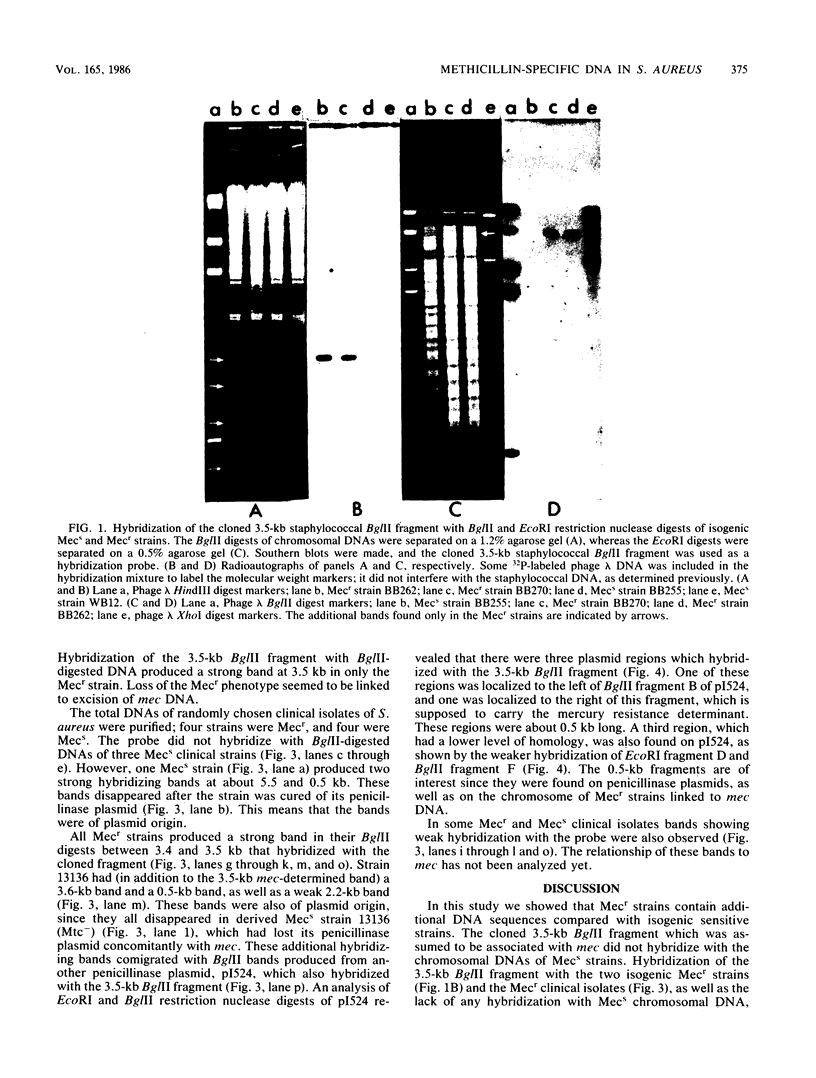
Abstract
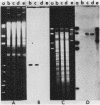
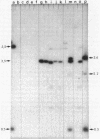
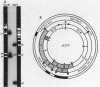
Additional DNA was shown to be present in methicillin-resistant Staphylococcus aureus by one- and two-dimensional restriction endonuclease analyses of the chromosomal DNA. A 3.5-kilobase Bg/II fragment, which was present in methicillin-resistant strains but not in the isogenic methicillin-sensitive parental strain, was cloned into newly constructed plasmid pWDB1 in Escherichia coli. Hybridization of this 3.5-kilobase Bg/II fragment with different methicillin-sensitive and methicillin-resistant S. aureus clinical isolates indicated that the fragment represents part of the methicillin resistance determinant (mec). In addition, the fragment carries a sequence that is present in some large staphylococcal plasmids, as well as in penicillinase plasmid pI524.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger-Bächi B. Increase in transduction efficiency of Tn551 mediated by the methicillin resistance marker. J Bacteriol. 1983 Apr;154(1):533–535. doi: 10.1128/jb.154.1.533-535.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger-Bächi B. Insertional inactivation of staphylococcal methicillin resistance by Tn551. J Bacteriol. 1983 Apr;154(1):479–487. doi: 10.1128/jb.154.1.479-487.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bächi B. Physical mapping of the BglI, BglII, PstI and EcoRI restriction fragments of staphylococcal phage phi 11 DNA. Mol Gen Genet. 1980;180(2):391–398. doi: 10.1007/BF00425853. [DOI] [PubMed] [Google Scholar]
- Cohen S., Sweeney H. M., Basu S. K. Mutations in prophage phi11 that impair the transducibility of their Staphylococcus aureus lysogens for methicillin resistance. J Bacteriol. 1977 Jan;129(1):237–245. doi: 10.1128/jb.129.1.237-245.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Hoopes B. C., McClure W. R. Studies on the selectivity of DNA precipitation by spermine. Nucleic Acids Res. 1981 Oct 24;9(20):5493–5504. doi: 10.1093/nar/9.20.5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser F. H., Benner E. J., Troy R., Hoeprich P. D. Experimental and clinical aspects of resistance determinants. Mode of resistance against beta-lactam antibiotics in staphylococci. Ann N Y Acad Sci. 1971 Jun 11;182:106–117. doi: 10.1111/j.1749-6632.1971.tb30649.x. [DOI] [PubMed] [Google Scholar]
- Kayser F. H. Die Resistenz methicillinresistenter Staphylokokken gegenüber neuen Cephalosporin-Antibiotika. Infection. 1980;8(4):165–170. doi: 10.1007/BF01639125. [DOI] [PubMed] [Google Scholar]
- Kieser T. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid. 1984 Jul;12(1):19–36. doi: 10.1016/0147-619x(84)90063-5. [DOI] [PubMed] [Google Scholar]
- Kuhl S. A., Pattee P. A., Baldwin J. N. Chromosomal map location of the methicillin resistance determinant in Staphylococcus aureus. J Bacteriol. 1978 Aug;135(2):460–465. doi: 10.1128/jb.135.2.460-465.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey R. W. Genetic control in methicillin-resistant strains of Staphylococcus aureus. J Med Microbiol. 1972 Nov;5(4):497–508. doi: 10.1099/00222615-5-4-497. [DOI] [PubMed] [Google Scholar]
- Murphy E., Novick R. P. Physical mapping of Staphylococcus aureus penicillinase plasmid pI524: characterization of an invertible region. Mol Gen Genet. 1979 Aug;175(1):19–30. doi: 10.1007/BF00267851. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Shafer W. M., Iandolo J. J. Genetics of staphylococcal enterotoxin B in methicillin-resistant isolates of Staphylococcus aureus. Infect Immun. 1979 Sep;25(3):902–911. doi: 10.1128/iai.25.3.902-911.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stewart G. C., Rosenblum E. D. Genetic behavior of the methicillin resistance determinant in Staphylococcus aureus. J Bacteriol. 1980 Dec;144(3):1200–1202. doi: 10.1128/jb.144.3.1200-1202.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee T., Inouye M. Two-dimensional DNA electrophoresis applied to the study of DNA methylation and the analysis of genome size in Myxococcus xanthus. J Mol Biol. 1982 Jan 15;154(2):181–196. doi: 10.1016/0022-2836(82)90059-6. [DOI] [PubMed] [Google Scholar]