Abstract
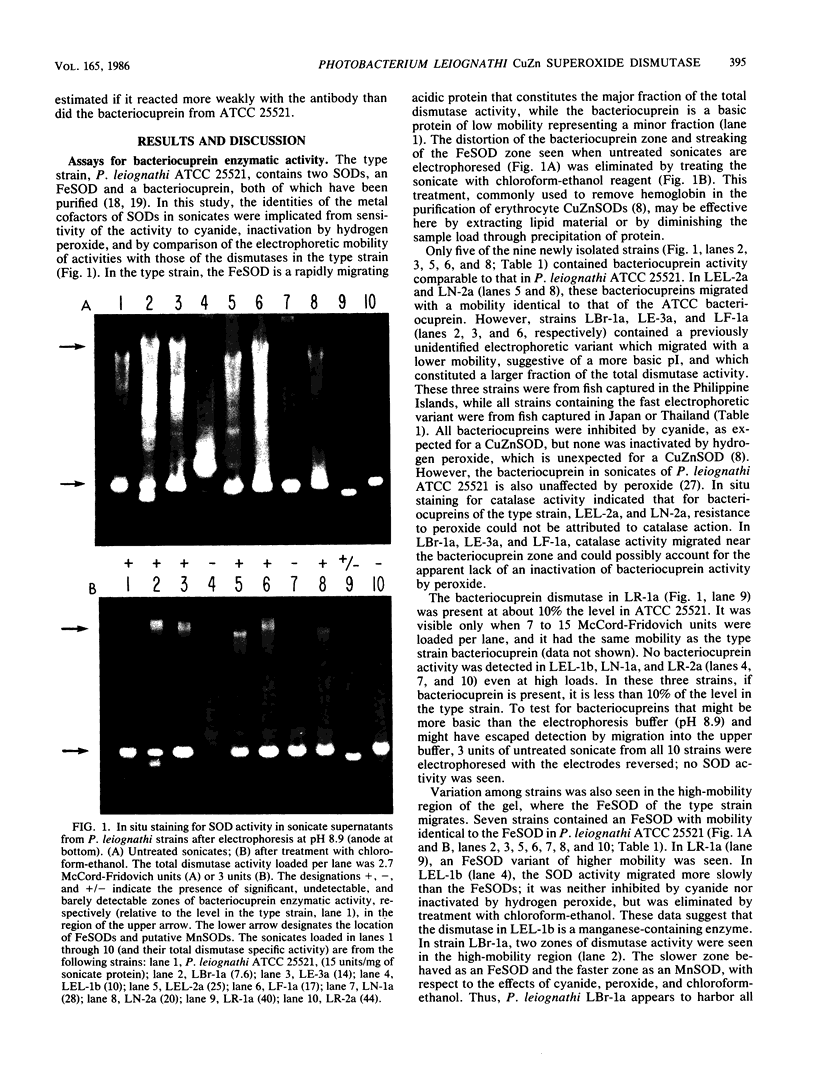
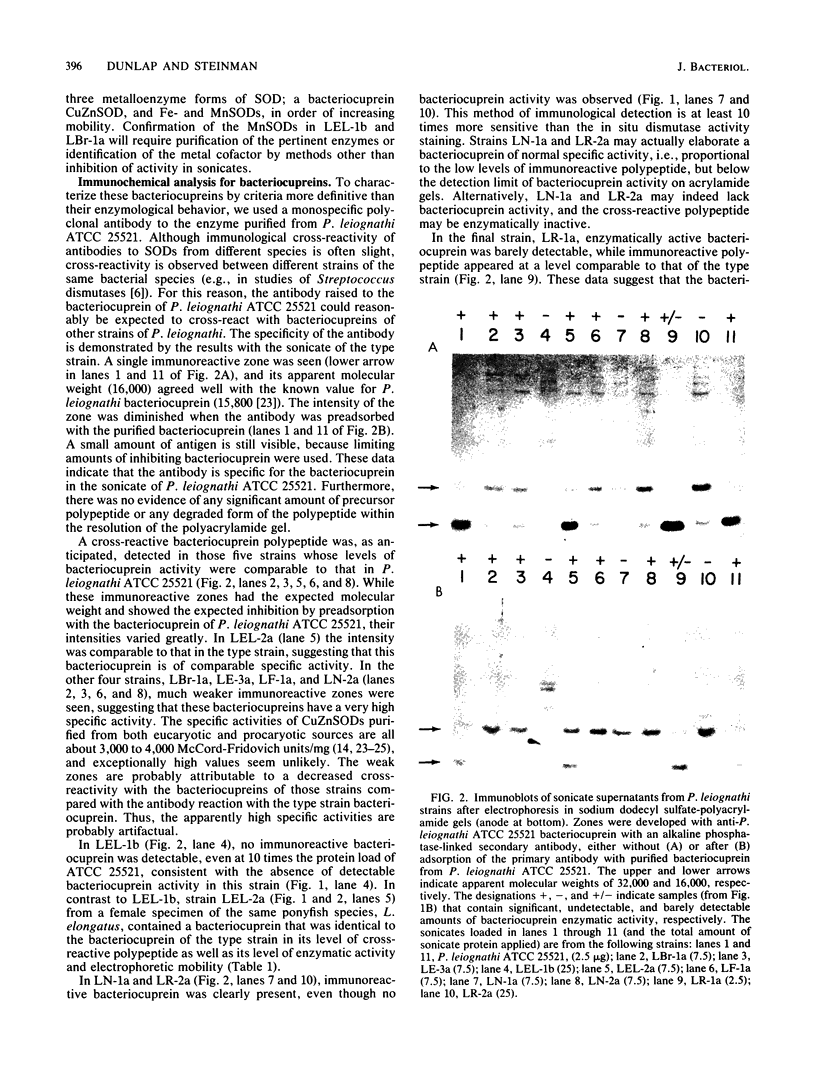
Photobacterium leiognathi ATCC 25521 (the type strain and light-organ symbiont of ponyfish) is one of the few bacteria that produces a copper-zinc superoxide dismutase, termed bacteriocuprein. We enzymologically and immunologically characterized the bacteriocuprein superoxide dismutases in sonicates from the type strain and nine additional strains of P. leiognathi, each isolated from the light organ of a separate ponyfish specimen, representing seven ponyfish species. The results indicate considerable strain variation. (i) The level of bacteriocuprein enzymatic activity varied greatly among strains from different species of ponyfish. In four of the nine strains, activity was low or undetectable, while in five strains it was comparable to that in the type strain. (ii) The bacteriocuprein in one strain had a specific activity much lower than that of the type strain, and in another strain, no bacteriocuprein activity and no cross-reactive polypeptide were detectable. (iii) A new electrophoretic variant, which migrated slower than that of strains from fish captured in Thailand and Japan, was identified in strains from fish captured in the Philippine Islands. (iv) Enzymological and immunological differences were observed in bacteriocupreins of strains from male and female specimens of the same ponyfish species, for the two species in which specimens of both sexes were examined. These observations raise the possibility that specific variations in the bacteriocupreins of P. leiognathi might be characteristic of the species, geographical source, or sex of the ponyfish host. Thus, the data indicate that the possibility of strain variation should be considered when other species are screened for bacteriocupreins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bannister J. V., Parker M. W. The presence of a copper/zinc superoxide dismutase in the bacterium Photobacterium leiognathi: a likely case of gene transfer from eukaryotes to prokaryotes. Proc Natl Acad Sci U S A. 1985 Jan;82(1):149–152. doi: 10.1073/pnas.82.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Britton L., Malinowski D. P., Fridovich I. Superoxide dismutase and oxygen metabolism in Streptococcus faecalis and comparisons with other organisms. J Bacteriol. 1978 Apr;134(1):229–236. doi: 10.1128/jb.134.1.229-236.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clare D. A., Duong M. N., Darr D., Archibald F., Fridovich I. Effects of molecular oxygen on detection of superoxide radical with nitroblue tetrazolium and on activity stains for catalase. Anal Biochem. 1984 Aug 1;140(2):532–537. doi: 10.1016/0003-2697(84)90204-5. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., McCord J. M., Fridovich I. Preparation and assay of superoxide dismutases. Methods Enzymol. 1978;53:382–393. doi: 10.1016/s0076-6879(78)53044-9. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Martin J. P., Jr, Fridovich I. Evidence for a natural gene transfer from the ponyfish to its bioluminescent bacterial symbiont Photobacter leiognathi. The close relationship between bacteriocuprein and the copper-zinc superoxide dismutase of teleost fishes. J Biol Chem. 1981 Jun 25;256(12):6080–6089. [PubMed] [Google Scholar]
- McFall-Ngai M. J., Dunlap P. V. External and internal sexual dimorphism in leiognathid fishes: morphological evidence for sex-specific bioluminescent signaling. J Morphol. 1984 Oct;182(1):71–83. doi: 10.1002/jmor.1051820105. [DOI] [PubMed] [Google Scholar]
- Puget K., Michelson A. M. Iron containing superoxide dismutases from luminous bacteria. Biochimie. 1974;56(9):1255–1267. doi: 10.1016/s0300-9084(74)80019-2. [DOI] [PubMed] [Google Scholar]
- Puget K., Michelson A. M. Isolation of a new copper-containing superoxide dismutase bacteriocuprein. Biochem Biophys Res Commun. 1974 Jun 4;58(3):830–838. doi: 10.1016/s0006-291x(74)80492-4. [DOI] [PubMed] [Google Scholar]
- Salin M. L., McCord J. M. Superoxide dismutases in polymorphonuclear leukocytes. J Clin Invest. 1974 Oct;54(4):1005–1009. doi: 10.1172/JCI107816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens G. J., Bannister J. V., Bannister W. H., Flohé L., Günzler W. A., Kim S. M., Otting F. The primary structure of Cu-Zn superoxide dismutase from Photobacterium leiognathi: evidence for a separate evolution of Cu-Zn superoxide dismutase in bacteria. Hoppe Seylers Z Physiol Chem. 1983 Jun;364(6):675–690. doi: 10.1515/bchm2.1983.364.1.675. [DOI] [PubMed] [Google Scholar]
- Steinman H. M. Bacteriocuprein superoxide dismutases in pseudomonads. J Bacteriol. 1985 Jun;162(3):1255–1260. doi: 10.1128/jb.162.3.1255-1260.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman H. M. Copper-zinc superoxide dismutase from Caulobacter crescentus CB15. A novel bacteriocuprein form of the enzyme. J Biol Chem. 1982 Sep 10;257(17):10283–10293. [PubMed] [Google Scholar]
- Vignais P. M., Terech A., Meyer C. M., Henry M. F. Isolation and characterization of a protein with cyanide-sensitive superoxide dismutase activity from the prokaryote, Paracoccus denitrificans. Biochim Biophys Acta. 1982 Mar 4;701(3):305–317. doi: 10.1016/0167-4838(82)90233-3. [DOI] [PubMed] [Google Scholar]