Abstract
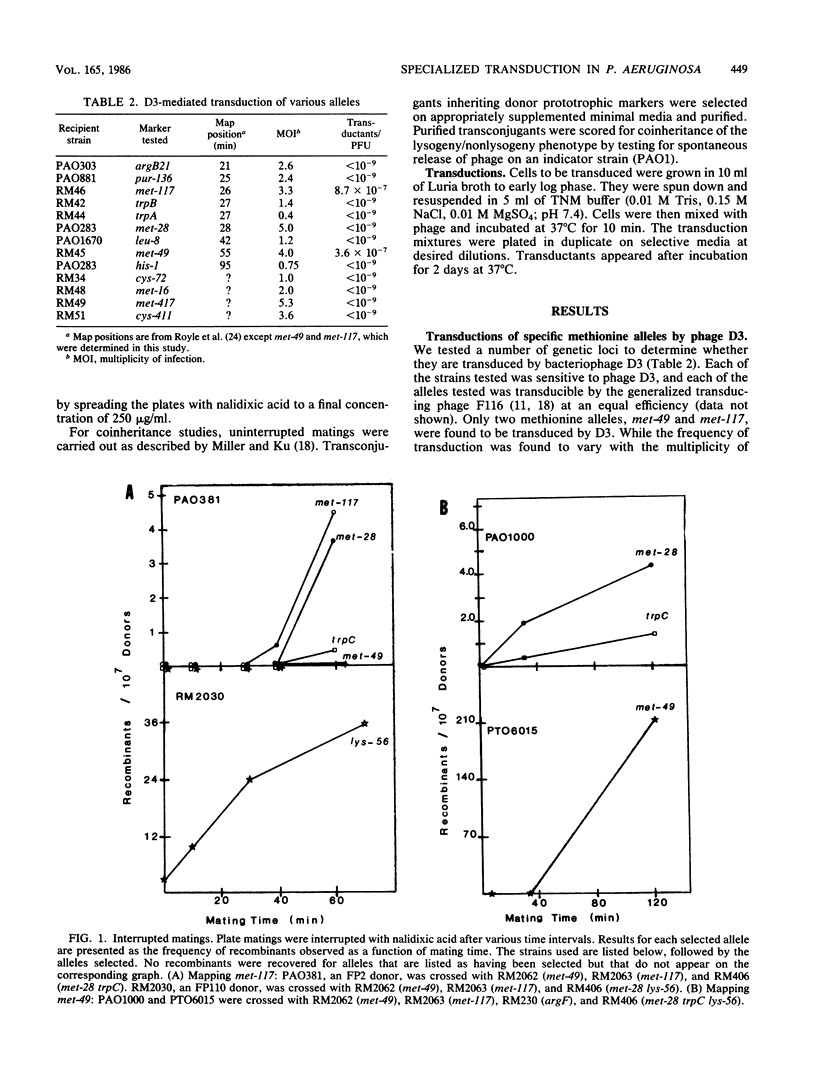
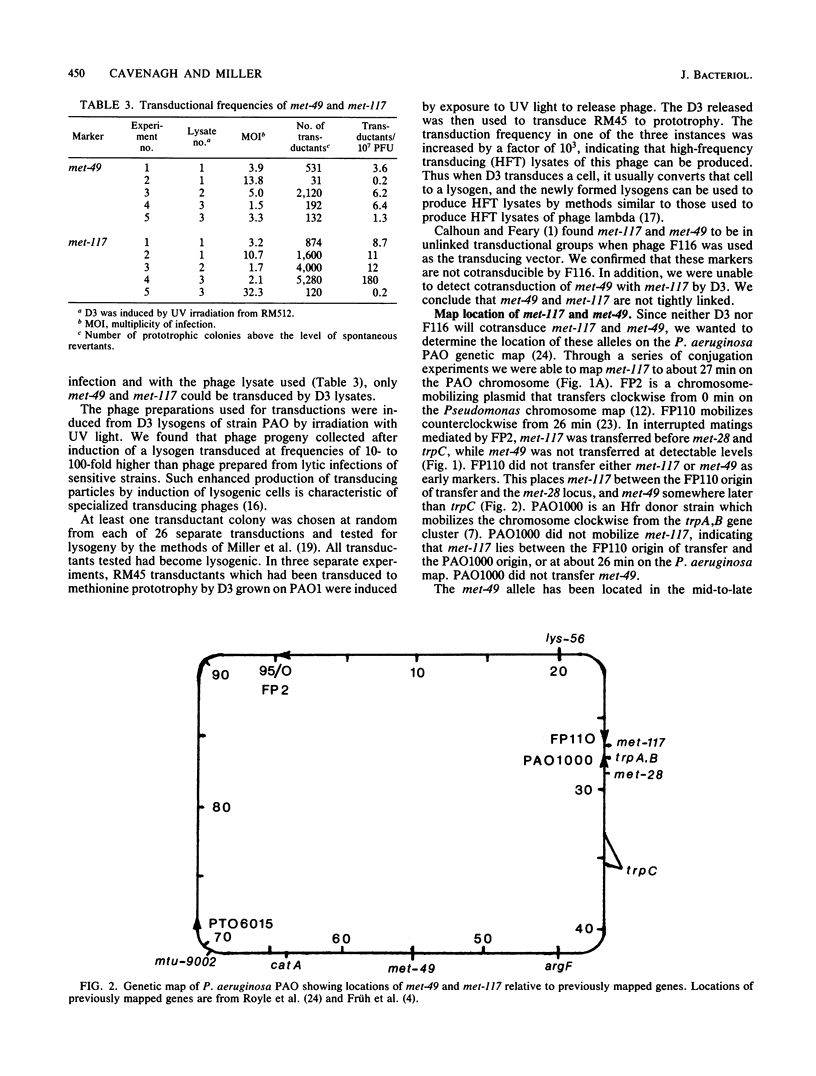
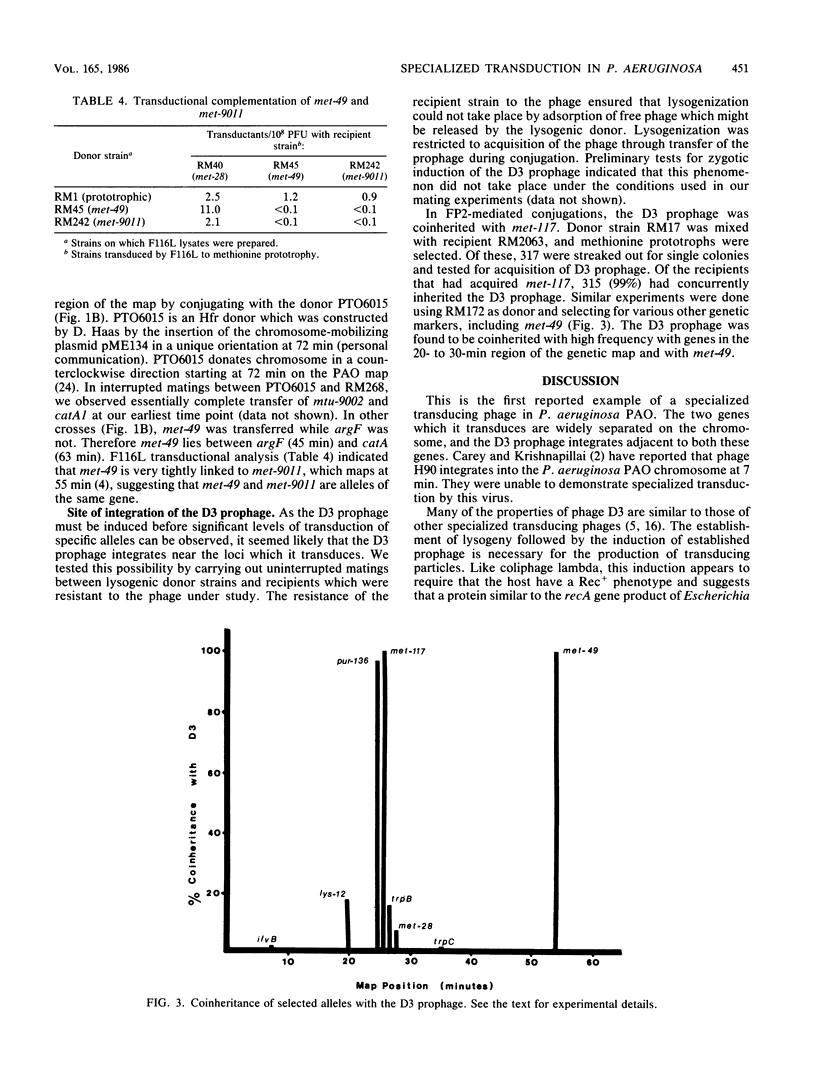
D3, a temperate bacteriophage of Pseudomonas aeruginosa PAO, was found to specifically transduce the alleles met-49 and met-117. Induction of established lysogens with UV light was necessary for the production of transducing lysates. Transduced cells were immune to superinfection by phage D3 and could give rise to high-frequency transducing lysates. Cotransduction of these two alleles could not be demonstrated. met-117 was mapped to 26 min on the PAO genetic map. Complementation studies using the generalized transducing phage F116L indicated that met-49 is an allele of met-9011 which maps at 55 min. The integrated D3 prophage was shown to be coinherited with met-117 and with met-49.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calhoun D. H., Feary T. W. Transductional analysis of Pseudomonas aeruginosa methionineless auxotrophs. J Bacteriol. 1969 Jan;97(1):210–216. doi: 10.1128/jb.97.1.210-216.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey K. E., Krishnapillai V. Location of prophage H90 on the chromosome of Pseudomonas aeruginosa strain PAO. Genet Res. 1974 Apr;23(2):155–164. doi: 10.1017/s0016672300014774. [DOI] [PubMed] [Google Scholar]
- EGAN J. B., HOLLOWAY B. W. Genetic studies on lysogeny in Pseudomonas aeruginosa. Aust J Exp Biol Med Sci. 1961 Feb;39:9–17. doi: 10.1038/icb.1961.2. [DOI] [PubMed] [Google Scholar]
- Früh R., Watson J. M., Haas D. Construction of recombination-deficient strains of Pseudomonas aeruginosa. Mol Gen Genet. 1983;191(2):334–337. doi: 10.1007/BF00334835. [DOI] [PubMed] [Google Scholar]
- HOLLOWAY B. W., EGAN J. B., MONK M. Lysogeny in Pseudomonas aeruginosa. Aust J Exp Biol Med Sci. 1960 Aug;38:321–329. doi: 10.1038/icb.1960.34. [DOI] [PubMed] [Google Scholar]
- HOLLOWAY B. W., JENNINGS P. A. An infectious fertility factor for Pseudomonas aeruginosa. Nature. 1958 Mar 22;181(4612):855–856. doi: 10.1038/181855b0. [DOI] [PubMed] [Google Scholar]
- Haas D., Holloway B. W. R factor variants with enhanced sex factor activity in Pseudomonas aeruginosa. Mol Gen Genet. 1976 Mar 30;144(3):243–251. doi: 10.1007/BF00341722. [DOI] [PubMed] [Google Scholar]
- Haas D., Watson J., Krieg R., Leisinger T. Isolation of an Hfr donor of Pseudomonas aeruginosa PAO by insertion of the plasmid RP1 into the tryptophan synthase gene. Mol Gen Genet. 1981;182(2):240–244. doi: 10.1007/BF00269664. [DOI] [PubMed] [Google Scholar]
- Holloway B. W. Genetics of Pseudomonas. Bacteriol Rev. 1969 Sep;33(3):419–443. doi: 10.1128/br.33.3.419-443.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway B. W. Mutants of Pseudomonas aeruginosa with reduced recombination ability. Mutat Res. 1966 Oct;3(5):452–455. doi: 10.1016/0027-5107(66)90055-8. [DOI] [PubMed] [Google Scholar]
- Kokjohn T. A., Miller R. V. Molecular cloning and characterization of the recA gene of Pseudomonas aeruginosa PAO. J Bacteriol. 1985 Aug;163(2):568–572. doi: 10.1128/jb.163.2.568-572.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. V., Ku C. M. Characterization of Pseudomonas aeruginosa mutants deficient in the establishment of lysogeny. J Bacteriol. 1978 Jun;134(3):875–883. doi: 10.1128/jb.134.3.875-883.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. V., Pemberton J. M., Clark A. J. Prophage F116: evidence for extrachromosomal location in Pseudomonas aeruginosa strain PAO. J Virol. 1977 Jun;22(3):844–847. doi: 10.1128/jvi.22.3.844-847.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. V., Pemberton J. M., Richards K. E. F116, D3 and G101: temperate bacteriophages of Pseudomonas aeruginosa. Virology. 1974 Jun;59(2):566–569. doi: 10.1016/0042-6822(74)90466-8. [DOI] [PubMed] [Google Scholar]
- Ornston L. N., Stanier R. Y. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. J Biol Chem. 1966 Aug 25;241(16):3776–3786. [PubMed] [Google Scholar]
- Pemberton J. M., Holloway B. W. Chromosome mapping in Pseudomonas aeruginosa. Genet Res. 1972 Jun;19(3):251–260. doi: 10.1017/s0016672300014518. [DOI] [PubMed] [Google Scholar]
- Royle P. L., Holloway B. W. Relationship between R and FP plasmids in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1980 Mar;17(3):293–297. doi: 10.1128/aac.17.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royle P. L., Matsumoto H., Holloway B. W. Genetic circularity of the Pseudomonas aeruginosa PAO chromosome. J Bacteriol. 1981 Jan;145(1):145–155. doi: 10.1128/jb.145.1.145-155.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Putte P., Holloway B. W. A thermosensitive recombination deficient mutant of Pseudomonas aeruginosa. Mutat Res. 1968 Sep-Oct;6(2):195–203. doi: 10.1016/0027-5107(68)90034-1. [DOI] [PubMed] [Google Scholar]


