Abstract
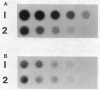
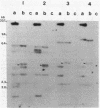
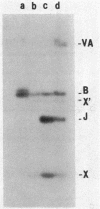
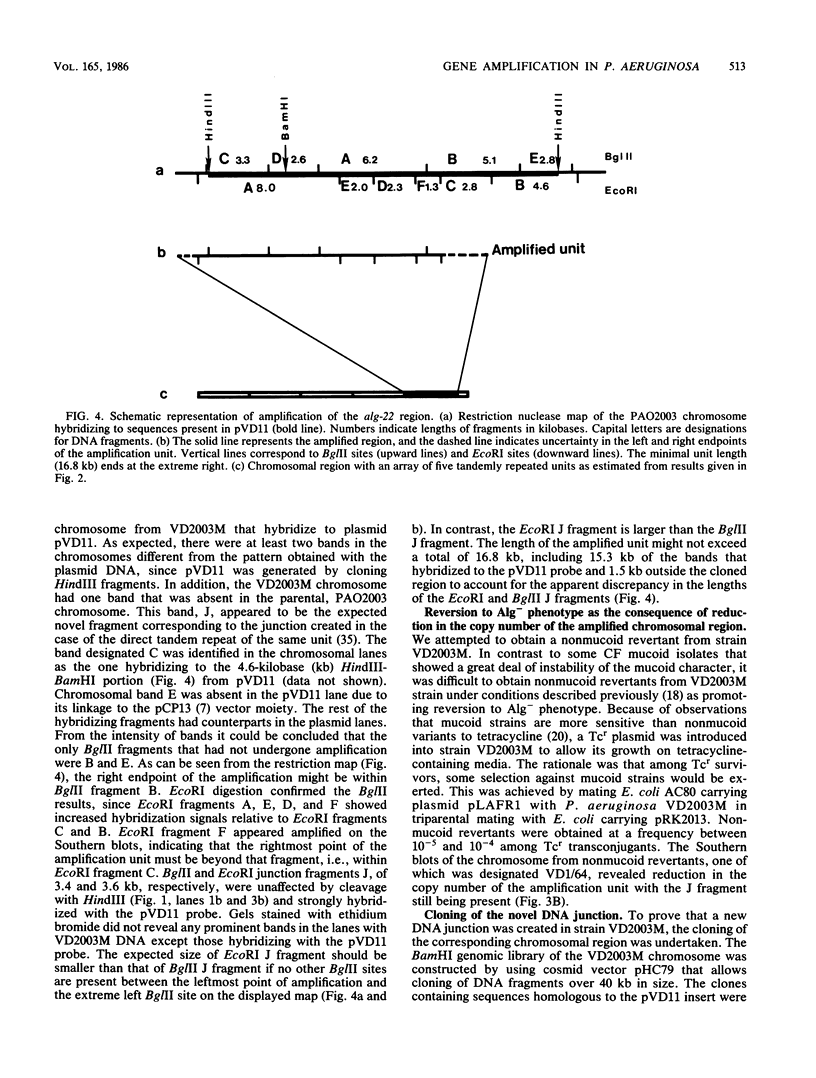
Gene amplification in the chromosome of rec-2 Pseudomonas aeruginosa PAO2003 upon growth on kanamycin-supplemented media led to a stable mucoid phenotype. The chromosomal region controlling alginate biosynthesis was shown to be amplified four to six times as a direct tandem repeat of at least 16.8 kilobase pairs. This amplification was deduced from Southern DNA-DNA hybridization patterns of the chromosomal DNA digested with restriction endonucleases BglII and EcoRI and probed with a cloned DNA segment complementing the alg-22 mutation. The part of the amplified unit carrying the novel DNA joint was cloned. The EcoRI junction fragment was further subcloned and used to probe chromosomes of parental strain PAO2003 and mucoid variant VD2003M. As predicted, the EcoRI junction fragment hybridized to the two chromosomal fragments required to produce the novel junction. Though the mucoid phenotype caused by gene amplification was stable, nonmucoid revertants were obtained at a low frequency on tetracycline-containing media. Southern hybridization of chromosomal DNA from a nonmucoid revertant revealed a reduction in the copy number of amplified DNA. These results suggest a direct relationship between amplification of this chromosomal segment and the induction of mucoidy.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. P., Roth J. R. Tandem chromosomal duplications in Salmonella typhimurium: fusion of histidine genes to novel promoters. J Mol Biol. 1978 Feb 15;119(1):147–166. doi: 10.1016/0022-2836(78)90274-7. [DOI] [PubMed] [Google Scholar]
- BRAMMAR W. J., CLARKE P. H. INDUCTION AND REPRESSION OF PSEUDOMONAS AERUGINOSA AMIDASE. J Gen Microbiol. 1964 Dec;37:307–319. doi: 10.1099/00221287-37-3-307. [DOI] [PubMed] [Google Scholar]
- Chakrabarty A. M., Friello D. A., Bopp L. H. Transposition of plasmid DNA segments specifying hydrocarbon degradation and their expression in various microorganisms. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3109–3112. doi: 10.1073/pnas.75.7.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler P. M., Krishnapillai V. Isolation and properties of recombination-deficient mutants of Pseudomonas aeruginosa. Mutat Res. 1974 Apr;23(1):15–23. doi: 10.1016/0027-5107(74)90155-9. [DOI] [PubMed] [Google Scholar]
- Chatterjee D. K., Kellogg S. T., Hamada S., Chakrabarty A. M. Plasmid specifying total degradation of 3-chlorobenzoate by a modified ortho pathway. J Bacteriol. 1981 May;146(2):639–646. doi: 10.1128/jb.146.2.639-646.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzins A., Chakrabarty A. M. Cloning of genes controlling alginate biosynthesis from a mucoid cystic fibrosis isolate of Pseudomonas aeruginosa. J Bacteriol. 1984 Jul;159(1):9–18. doi: 10.1128/jb.159.1.9-18.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzins A., Nixon L. L., Vanags R. I., Chakrabarty A. M. Cloning of Escherichia coli and Pseudomonas aeruginosa phosphomannose isomerase genes and their expression in alginate-negative mutants of Pseudomonas aeruginosa. J Bacteriol. 1985 Jan;161(1):249–257. doi: 10.1128/jb.161.1.249-257.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis P. B., di Sant'Agnese P. A. A review. Cystic fibrosis at forty--quo vadis? Pediatr Res. 1980 Feb;14(2):83–87. doi: 10.1203/00006450-198002000-00002. [DOI] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Emmons S. W., MacCosham V., Baldwin R. L. Tandem genetic duplications in phage lambda. III. The frequency of duplication mutants in two derivatives of phage lambda is independent of known recombination systems. J Mol Biol. 1975 Jan 15;91(2):133–146. doi: 10.1016/0022-2836(75)90154-0. [DOI] [PubMed] [Google Scholar]
- Evans L. R., Linker A. Production and characterization of the slime polysaccharide of Pseudomonas aeruginosa. J Bacteriol. 1973 Nov;116(2):915–924. doi: 10.1128/jb.116.2.915-924.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A. M., Long S. R., Brown S. E., Buikema W. J., Ausubel F. M. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene. 1982 Jun;18(3):289–296. doi: 10.1016/0378-1119(82)90167-6. [DOI] [PubMed] [Google Scholar]
- Fyfe J. A., Govan J. R. Alginate synthesis in mucoid Pseudomonas aeruginosa: a chromosomal locus involved in control. J Gen Microbiol. 1980 Aug;119(2):443–450. doi: 10.1099/00221287-119-2-443. [DOI] [PubMed] [Google Scholar]
- Goldberg J. B., Ohman D. E. Cloning and expression in Pseudomonas aeruginosa of a gene involved in the production of alginate. J Bacteriol. 1984 Jun;158(3):1115–1121. doi: 10.1128/jb.158.3.1115-1121.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govan J. R. Antibiotic therapy and cystic fibrosis: increased resistance of mucoid Pseudomonas aeruginosa to carbenicillin. J Antimicrob Chemother. 1976 Jun;2(2):215–217. doi: 10.1093/jac/2.2.215. [DOI] [PubMed] [Google Scholar]
- Govan J. R., Fyfe J. A. Mucoid Pseudomonas aeruginosa and cystic fibrosis: resistance of the mucoid from to carbenicillin, flucloxacillin and tobramycin and the isolation of mucoid variants in vitro. J Antimicrob Chemother. 1978 May;4(3):233–240. doi: 10.1093/jac/4.3.233. [DOI] [PubMed] [Google Scholar]
- Govan J. R. Mucoid strains of Pseudomonas aeruginosa: the influence of culture medium on the stability of mucus production. J Med Microbiol. 1975 Nov;8(4):513–522. doi: 10.1099/00222615-8-4-513. [DOI] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- IACOCCA V. F., SIBINGA M., BARBERO G. J. RESPIRATORY TRACT BACTERIOLOGY IN CYSTIC FIBROSIS. Am J Dis Child. 1963 Sep;106:315–324. doi: 10.1001/archpedi.1963.02080050317012. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbourn J. P. Infection in cystic fibrosis. Lancet. 1970 Oct 24;2(7678):878–879. doi: 10.1016/s0140-6736(70)92050-7. [DOI] [PubMed] [Google Scholar]
- Knutson C. A., Jeanes A. A new modification of the carbazole analysis: application to heteropolysaccharides. Anal Biochem. 1968 Sep;24(3):470–481. doi: 10.1016/0003-2697(68)90154-1. [DOI] [PubMed] [Google Scholar]
- LINKER A., JONES R. S. A POLYSACCHARIDE RESEMBLING ALGINIC ACID FROM A PSEUDOMONAS MICRO-ORGANISM. Nature. 1964 Oct 10;204:187–188. doi: 10.1038/204187a0. [DOI] [PubMed] [Google Scholar]
- Marks M. I., Prentice R., Swarson R., Cotton E. K., Eickhoff T. C. Carbenicillin and gentamicin: pharmacologic studies in patients with cystic fibrosis and pseudomonas pulmonary infections. J Pediatr. 1971 Nov;79(5):822–828. doi: 10.1016/s0022-3476(71)80401-8. [DOI] [PubMed] [Google Scholar]
- Martin D. R. Mucoid variation in Pseudomonas aeruginosa induced by the action of phage. J Med Microbiol. 1973 Feb;6(1):111–118. doi: 10.1099/00222615-6-1-111. [DOI] [PubMed] [Google Scholar]
- Ohman D. E., West M. A., Flynn J. L., Goldberg J. B. Method for gene replacement in Pseudomonas aeruginosa used in construction of recA mutants: recA-independent instability of alginate production. J Bacteriol. 1985 Jun;162(3):1068–1074. doi: 10.1128/jb.162.3.1068-1074.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poustka A., Rackwitz H. R., Frischauf A. M., Hohn B., Lehrach H. Selective isolation of cosmid clones by homologous recombination in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4129–4133. doi: 10.1073/pnas.81.13.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. A general method for site-directed mutagenesis in prokaryotes. Nature. 1981 Jan 1;289(5793):85–88. doi: 10.1038/289085a0. [DOI] [PubMed] [Google Scholar]
- Tlsty T. D., Albertini A. M., Miller J. H. Gene amplification in the lac region of E. coli. Cell. 1984 May;37(1):217–224. doi: 10.1016/0092-8674(84)90317-9. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]