Abstract
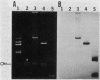
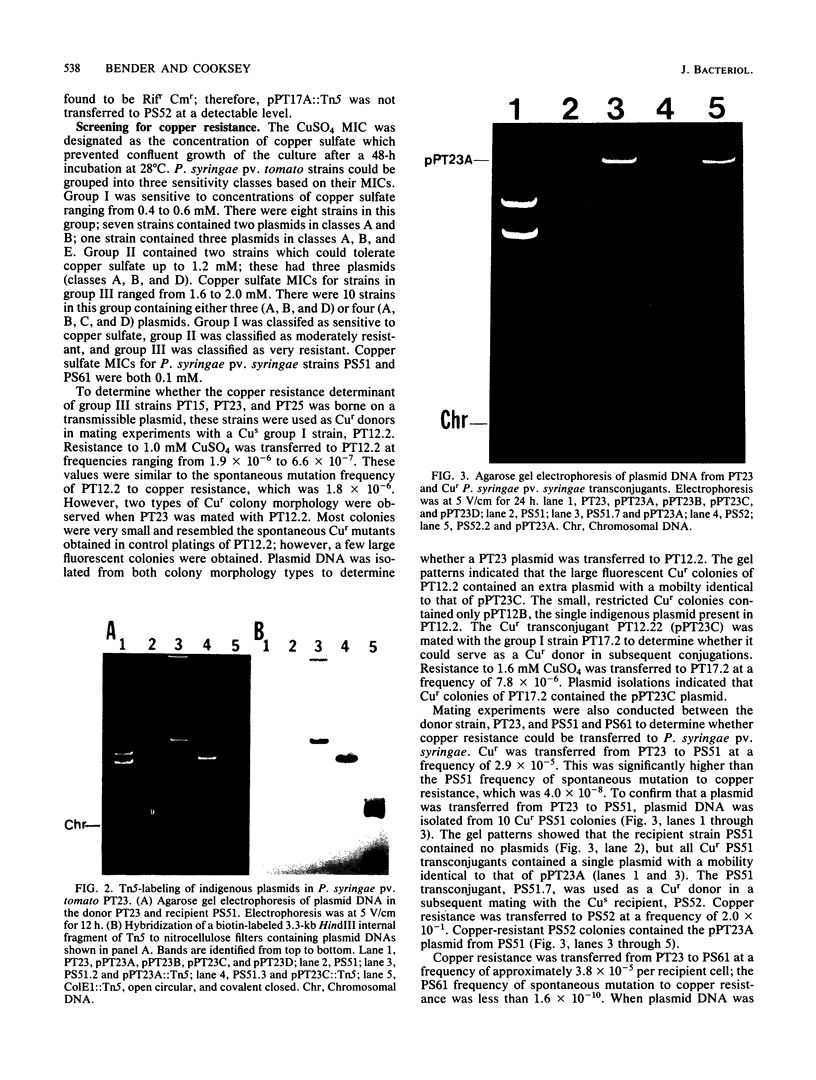
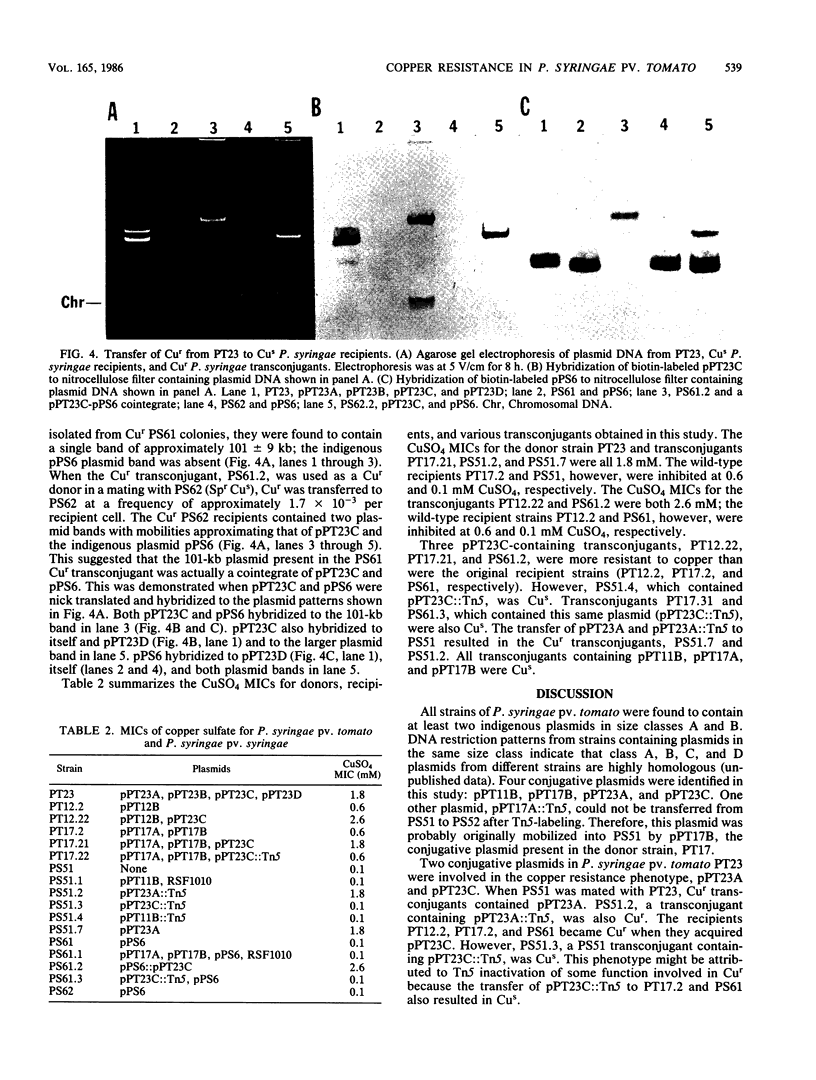
Twenty strains of Pseudomonas syringae pv. tomato were examined for the presence of plasmid DNA. P. syringae pv. tomato plasmids were grouped into five size classes: class A ranged from 95 to 103 kilobases (kb); class B ranged from 71 to 83 kb; class C ranged from 59 to 67 kb; class D ranged from 37 to 39 kb; and class E was 29 kb. All strains contained at least two plasmids in classes A and B. The conjugative ability of P. syringae pv. tomato plasmids in three strains was demonstrated by mobilization of the nonconjugative plasmid RSF1010 into Pseudomonas syringae pv. syringae recipients. Plasmids from the three conjugative strains were labeled with Tn5. Four conjugative plasmids were identified by their repeated transfer to P. syringae pv. syringae recipients. P. syringae pv. tomato strains varied in sensitivity to copper sulfate (CuSO4): MICs were 0.4 to 0.6 mM for sensitive strains, 1.2 mM for moderately resistant strains, and 1.6 to 2.0 mM for very resistant strains. One very resistant strain, PT23, functioned as a donor of copper resistance. Recipient P. syringae pv. syringae strains PS51 and PS61 were inhibited by 0.1 mM CuSO4, whereas the CuSO4 MICs for transconjugant strains PS51(pPT23A) and PS61(pPT23C) were 1.8 and 2.6 mM, respectively. P. syringae pv. tomato strains PT12.2 and PT17.2 were inhibited by 0.6 mM copper sulfate, but their copper sulfate MICs were 2.6 and 1.8 mM, respectively, when they acquired pPT23C. Therefore, copper resistance in PT23 was controlled by two conjugative plasmids, designated pPT23A (101 kb) and pPT23C (67 kb).
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calomiris J. J., Armstrong J. L., Seidler R. J. Association of metal tolerance with multiple antibiotic resistance of bacteria isolated from drinking water. Appl Environ Microbiol. 1984 Jun;47(6):1238–1242. doi: 10.1128/aem.47.6.1238-1242.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comai L., Kosuge T. Involvement of plasmid deoxyribonucleic acid in indoleacetic acid synthesis in Pseudomonas savastanoi. J Bacteriol. 1980 Aug;143(2):950–957. doi: 10.1128/jb.143.2.950-957.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouse G. F., Frischauf A., Lehrach H. An integrated and simplified approach to cloning into plasmids and single-stranded phages. Methods Enzymol. 1983;101:78–89. doi: 10.1016/0076-6879(83)01006-x. [DOI] [PubMed] [Google Scholar]
- Dutta G. N., Devriese L. A. Sensitivity and resistance to growth promoting agents in animal lactobacilli. J Appl Bacteriol. 1981 Oct;51(2):283–288. doi: 10.1111/j.1365-2672.1981.tb01243.x. [DOI] [PubMed] [Google Scholar]
- Elek S. D., Higney L. Resistogram typing--a new epidemiological tool: application to Escherichia coli. J Med Microbiol. 1970 Feb;3(1):103–110. doi: 10.1099/00222615-3-1-103. [DOI] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel S., Welch J. W. Tandem gene amplification mediates copper resistance in yeast. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5342–5346. doi: 10.1073/pnas.79.17.5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T. J. Plasmid-determined resistance to antimicrobial drugs and toxic metal ions in bacteria. Microbiol Rev. 1983 Sep;47(3):361–409. doi: 10.1128/mr.47.3.361-409.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., van Embden J., Falkow S. Molecular nature of two nonconjugative plasmids carrying drug resistance genes. J Bacteriol. 1974 Feb;117(2):619–630. doi: 10.1128/jb.117.2.619-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara M., Kamio Y., Terawaki Y. Cupric ion resistance as a new genetic marker of a temperature sensitive R plasmid, Rtsl in Escherichia coli. Biochem Biophys Res Commun. 1978 May 15;82(1):74–80. doi: 10.1016/0006-291x(78)90578-8. [DOI] [PubMed] [Google Scholar]
- Jahn G., Laufs R., Kaulfers P. M., Kolenda H. Molecular nature of two Haemophilus influenzae R factors containing resistances and the multiple integration of drug resistance transposons. J Bacteriol. 1979 May;138(2):584–597. doi: 10.1128/jb.138.2.584-597.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen R. A., Rothstein S. J., Reznikoff W. S. A restriction enzyme cleavage map of Tn5 and location of a region encoding neomycin resistance. Mol Gen Genet. 1979;177(1):65–72. doi: 10.1007/BF00267254. [DOI] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Piwowarski J. M., Shaw P. D. Characterization of plasmids from plant pathogenic pseudomonads. Plasmid. 1982 Jan;7(1):85–94. doi: 10.1016/0147-619x(82)90030-0. [DOI] [PubMed] [Google Scholar]
- Selvaraj G., Iyer V. N. Suicide plasmid vehicles for insertion mutagenesis in Rhizobium meliloti and related bacteria. J Bacteriol. 1983 Dec;156(3):1292–1300. doi: 10.1128/jb.156.3.1292-1300.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers A. O., Silver S. Microbial transformations of metals. Annu Rev Microbiol. 1978;32:637–672. doi: 10.1146/annurev.mi.32.100178.003225. [DOI] [PubMed] [Google Scholar]
- Terawaki Y., Takayasu H., Akiba T. Thermosensitive replication of a kanamycin resistance factor. J Bacteriol. 1967 Sep;94(3):687–690. doi: 10.1128/jb.94.3.687-690.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetaz T. J., Luke R. K. Plasmid-controlled resistance to copper in Escherichia coli. J Bacteriol. 1983 Jun;154(3):1263–1268. doi: 10.1128/jb.154.3.1263-1268.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma M. M., Thomas W. A., Prasad C. Resistance to inorganic salts and antibiotics among sewage-borne Enterobacteriaceae and Achromobacteriaceae. J Appl Bacteriol. 1976 Oct;41(2):347–349. doi: 10.1111/j.1365-2672.1976.tb00643.x. [DOI] [PubMed] [Google Scholar]
- Wood W. B. Host specificity of DNA produced by Escherichia coli: bacterial mutations affecting the restriction and modification of DNA. J Mol Biol. 1966 Mar;16(1):118–133. doi: 10.1016/s0022-2836(66)80267-x. [DOI] [PubMed] [Google Scholar]