Abstract
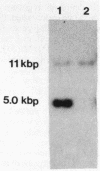
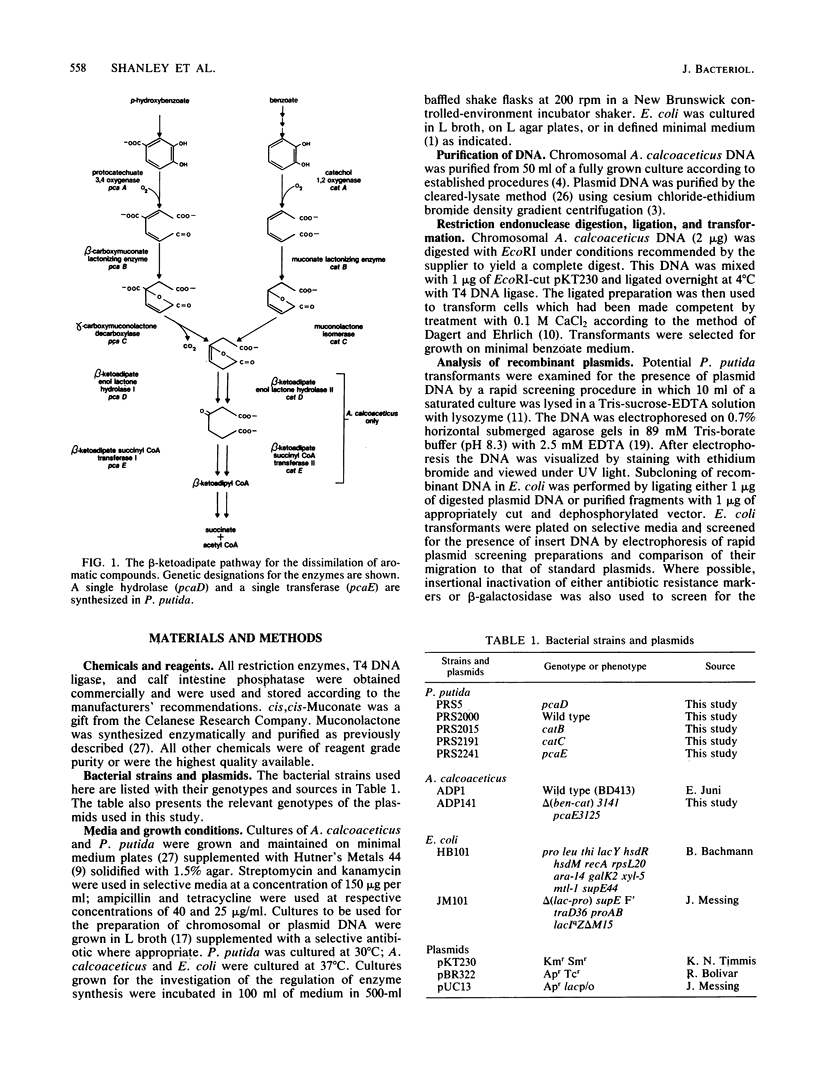
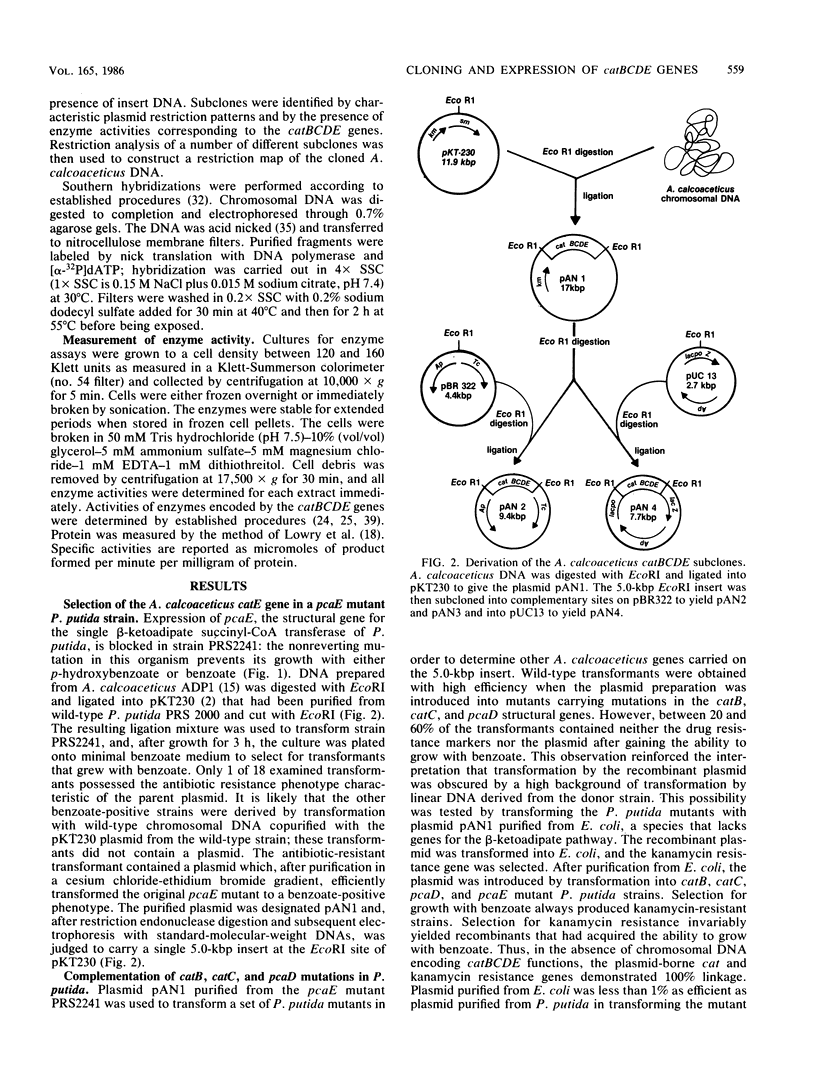
This report describes the isolation and preliminary characterization of a 5.0-kilobase-pair (kbp) EcoRI DNA restriction fragment carrying the catBCDE genes from Acinetobacter calcoaceticus. The respective genes encode enzymes that catalyze four consecutive reactions in the catechol branch of the beta-ketoadipate pathway: catB, muconate lactonizing enzyme (EC 5.5.1.1); catC, muconolactone isomerase (EC 5.3.3.4); catD, beta-ketoadipate enol-lactone hydrolase (EC 3.1.1.24); and catE, beta-ketoadipate succinyl-coenzyme A transferase (EC 2.8.3.6). In A. calcoaceticus, pcaDE genes encode products with the same enzyme activities as those encoded by the respective catDE genes. In Pseudomonas putida, the requirements for both catDE and pcaDE genes are met by a single set of genes, designated pcaDE. A P. putida mutant with a dysfunctional pcaE gene was used to select a recombinant pKT230 plasmid carrying the 5.0-kbp EcoRI restriction fragment containing the A. calcoaceticus catE structural gene. The recombinant plasmid, pAN1, complemented P. putida mutants with lesions in catB, catC, pcaD, and pcaE genes; the complemented activities were expressed constitutively in the recombinant P. putida strains. After introduction into Escherichia coli, the pAN1 plasmid expressed the activities constitutively but at much lower levels that those found in the P. putida transformants or in fully induced cultures of A. calcoaceticus or P. putida. When placed under the control of a lac promoter on a recombinant pUC13 plasmid in E. coli, the A. calcoaceticus restriction fragment expressed catBCDE activities at levels severalfold higher than those found in fully induced cultures of A. calcoaceticus. Thus there is no translational barrier to expression of the A. calcoaceticus genes at high levels in E. coli. The genetic origin of the cloned catBCDE genes was demonstrated by the fact that the 5.0-kbp EcoRI restriction fragment hybridized with a corresponding fragment from wild-type A. calcoaceticus DNA. This fragment was missing in DNA from an A. calcoaceticus mutant in which the cat genes had been removed by deletion. The properties of the cloned fragment demonstrate physical linkage of the catBCDE genes and suggest that they are coordinately transcribed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. H. Growth Requirements of Virus-Resistant Mutants of Escherichia Coli Strain "B". Proc Natl Acad Sci U S A. 1946 May;32(5):120–128. doi: 10.1073/pnas.32.5.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNS K. I., THOMAS C. A., Jr ISOLATION OF HIGH MOLECULAR WEIGHT DNA FROM HEMOPHILUS INFLUENZAE. J Mol Biol. 1965 Mar;11:476–490. doi: 10.1016/s0022-2836(65)80004-3. [DOI] [PubMed] [Google Scholar]
- Bagdasarian M., Lurz R., Rückert B., Franklin F. C., Bagdasarian M. M., Frey J., Timmis K. N. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981 Dec;16(1-3):237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Circular DNA forms of colicinogenic factors E1, E2 and E3 from Escherichia coli. J Mol Biol. 1968 Sep 14;36(2):185–194. doi: 10.1016/0022-2836(68)90374-4. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Betlach M. C., Boyer H. W. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2(2):75–93. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Clarke P. H., Laverack P. D. Expression of the argF gene of Pseudomonas aeruginosa in Pseudomonas aeruginosa, Pseudomonas putida, and Escherichia coli. J Bacteriol. 1983 Apr;154(1):508–512. doi: 10.1128/jb.154.1.508-512.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cánovas J. L., Johnson B. F. Regulation of the enzymes of the beta-ketoadipate pathway in Moraxella calcoacetica. 4. Constitutive synthesis of beta-ketoadipate succinyl-CoA transferases II and 3. Eur J Biochem. 1968 Jan;3(3):312–317. doi: 10.1111/j.1432-1033.1968.tb19531.x. [DOI] [PubMed] [Google Scholar]
- Cánovas J. L., Stanier R. Y. Regulation of the enzymes of the beta-ketoadipate pathway in Moraxella calcoacetica. 1. General aspects. Eur J Biochem. 1967 May;1(3):289–300. doi: 10.1007/978-3-662-25813-2_40. [DOI] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Ensley B. D., Ratzkin B. J., Osslund T. D., Simon M. J., Wackett L. P., Gibson D. T. Expression of naphthalene oxidation genes in Escherichia coli results in the biosynthesis of indigo. Science. 1983 Oct 14;222(4620):167–169. doi: 10.1126/science.6353574. [DOI] [PubMed] [Google Scholar]
- Franklin F. C., Bagdasarian M., Bagdasarian M. M., Timmis K. N. Molecular and functional analysis of the TOL plasmid pWWO from Pseudomonas putida and cloning of genes for the entire regulated aromatic ring meta cleavage pathway. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7458–7462. doi: 10.1073/pnas.78.12.7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Ebina Y., Nakazawa A., Nakazawa T. Nucleotide sequence surrounding transcription initiation site of xylABC operon on TOL plasmid of Pseudomonas putida. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1688–1691. doi: 10.1073/pnas.81.6.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juni E. Interspecies transformation of Acinetobacter: genetic evidence for a ubiquitous genus. J Bacteriol. 1972 Nov;112(2):917–931. doi: 10.1128/jb.112.2.917-931.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L., Kingsbury D. T., Helinski D. R. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. doi: 10.1128/jb.114.2.577-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCorkle G. M., Yeh W. K., Fletcher P., Ornston L. N. Repetitions in the NH2-terminal amino acid sequence of beta-ketoadipate enol-lactone hydrolase from Pseudomonas putida. J Biol Chem. 1980 Jul 10;255(13):6335–6341. [PubMed] [Google Scholar]
- Mergeay M., Boyen A., Legrain C., Glansdorff N. Expression of Escherichia coli K-12 arginine genes in Pseudomonas fluorescens. J Bacteriol. 1978 Dec;136(3):1187–1188. doi: 10.1128/jb.136.3.1187-1188.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton N. P., Atkinson T., Sherwood R. F. Molecular cloning of the Pseudomonas carboxypeptidase G2 gene and its expression in Escherichia coli and Pseudomonas putida. J Bacteriol. 1983 Dec;156(3):1222–1227. doi: 10.1128/jb.156.3.1222-1227.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylroie J. R., Friello D. A., Chakrabarty A. M. Transformation of Pseudomonas putida with chromosomal DNA. Biochem Biophys Res Commun. 1978 May 15;82(1):281–288. doi: 10.1016/0006-291x(78)90606-x. [DOI] [PubMed] [Google Scholar]
- Ornston L. N. Regulation of catabolic pathways in Pseudomonas. Bacteriol Rev. 1971 Jun;35(2):87–116. doi: 10.1128/br.35.2.87-116.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornston L. N., Stanier R. Y. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. J Biol Chem. 1966 Aug 25;241(16):3776–3786. [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. 3. Enzymes of the catechol pathway. J Biol Chem. 1966 Aug 25;241(16):3795–3799. [PubMed] [Google Scholar]
- Parke D., Ornston L. N. Constitutive synthesis of enzymes of the protocatechuate pathway and of the beta-ketoadipate uptake system in mutant strains of Pseudomonas putida. J Bacteriol. 1976 Apr;126(1):272–281. doi: 10.1128/jb.126.1.272-281.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. N., Mazumdar S., Ornston L. N. Beta-ketoadipate enol-lactone hydrolases I and II from Acinetobacter calcoaceticus. J Biol Chem. 1975 Aug 25;250(16):6567–6567. [PubMed] [Google Scholar]
- Schell M. A. Cloning and expression in Escherichia coli of the naphthalene degradation genes from plasmid NAH7. J Bacteriol. 1983 Feb;153(2):822–829. doi: 10.1128/jb.153.2.822-829.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Ornston L. N. The beta-ketoadipate pathway. Adv Microb Physiol. 1973;9(0):89–151. [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelis M. L., Ornston L. N. Genetic control of enzyme induction in the -ketoadipate pathway of Pseudomonas putida: deletion mapping of cat mutations. J Bacteriol. 1972 Feb;109(2):790–795. doi: 10.1128/jb.109.2.790-795.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh W. K., Fletcher P., Ornston L. N. Evolutionary divergence of co-selected beta-ketoadipate enol-lactone hydrolases in Acinetobacter calcoaceticus. J Biol Chem. 1980 Jul 10;255(13):6342–6346. [PubMed] [Google Scholar]
- Yeh W. K., Fletcher P., Ornston N. Homologies in the NH2-terminal amino acid sequences of gamma-carboxymuconolactone decarboxylases and muconolactone isomerases. J Biol Chem. 1980 Jul 10;255(13):6347–6354. [PubMed] [Google Scholar]
- Yeh W. K., Ornston L. N. Evolutionarily homologous alpha 2 beta 2 oligomeric structures in beta-ketoadipate succinyl-CoA transferases from Acinetobacter calcoaceticus and Pseudomonas putida. J Biol Chem. 1981 Feb 25;256(4):1565–1569. [PubMed] [Google Scholar]