Abstract
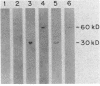
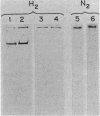
Two polypeptides present in aerobic and anaerobic cultures of Escherichia coli HB101 were shown to cross-react with antibodies to the 30- and 60-kilodalton (kDa) subunits of the uptake hydrogenase of Rhizobium japonicum. The cross-reactive polypeptides in a series of different E. coli strains are of Mrs ca. 60,000 and 30,000, and both polypeptides are present in proportion to measurable hydrogen uptake (Hup) activity (r = 0.95). The 60-kDa polypeptide from E. coli HB101 comigrated on native gels with detectable Hup activity. The exact role of the 30-kDa polypeptide in E. coli is unclear. E. coli MBM7061, a natural Hup- variant, grown anaerobically or aerobically lacked detectable Hup activity and failed to cross-react with the antisera against the hydrogenase from R. japonicum. Anaerobically cultured E. coli MBM7061, however, did express formate hydrogenlyase activity, indicating that the hydrogenases involved in the oxygen-dependent activation of hydrogen and the formate-dependent evolution of hydrogen are biochemically distinct.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. W., Hall D. O. Purification of the membrane-bound hydrogenase of Escherichia coli. Biochem J. 1979 Oct 1;183(1):11–22. doi: 10.1042/bj1830011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams M. W., Mortenson L. E., Chen J. S. Hydrogenase. Biochim Biophys Acta. 1980 Dec;594(2-3):105–176. doi: 10.1016/0304-4173(80)90007-5. [DOI] [PubMed] [Google Scholar]
- Arp D. J. Rhizobium japonicum hydrogenase: purification to homogeneity from soybean nodules, and molecular characterization. Arch Biochem Biophys. 1985 Mar;237(2):504–512. doi: 10.1016/0003-9861(85)90303-0. [DOI] [PubMed] [Google Scholar]
- Ballantine S. P., Boxer D. H. Nickel-containing hydrogenase isoenzymes from anaerobically grown Escherichia coli K-12. J Bacteriol. 1985 Aug;163(2):454–459. doi: 10.1128/jb.163.2.454-459.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell M. A., Haugland R. A., Evans H. J. Construction of a Rhizobium japonicum gene bank and use in isolation of a hydrogen uptake gene. Proc Natl Acad Sci U S A. 1983 Jan;80(1):181–185. doi: 10.1073/pnas.80.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- GRAY C. T., GEST H. BIOLOGICAL FORMATION OF MOLECULAR HYDROGEN. Science. 1965 Apr 9;148(3667):186–192. doi: 10.1126/science.148.3667.186. [DOI] [PubMed] [Google Scholar]
- Glick B. R., Wang P. Y., Schneider H., Martin W. G. Identification and partial characterization of an Escherichia coli mutant with altered hydrogenase activity. Can J Biochem. 1980 Apr;58(4):361–367. doi: 10.1139/o80-047. [DOI] [PubMed] [Google Scholar]
- Hanus F. J., Maier R. J., Evans H. J. Autotrophic growth of H2-uptake-positive strains of Rhizobium japonicum in an atmosphere supplied with hydrogen gas. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1788–1792. doi: 10.1073/pnas.76.4.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker A. R., Lambert G. R., Hanus F. J., Evans H. J. Further evidence that two unique subunits are essential for expression of hydrogenase activity in Rhizobium japonicum. J Bacteriol. 1985 Oct;164(1):187–191. doi: 10.1128/jb.164.1.187-191.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker A. R., Xu L. S., Hanus F. J., Evans H. J. Some properties of the nickel-containing hydrogenase of chemolithotrophically grown Rhizobium japonicum. J Bacteriol. 1984 Sep;159(3):850–856. doi: 10.1128/jb.159.3.850-856.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugland R. A., Hanus F. J., Cantrell M. A., Evans H. J. Rapid Colony Screening Method for Identifying Hydrogenase Activity in Rhizobium japonicum. Appl Environ Microbiol. 1983 Mar;45(3):892–897. doi: 10.1128/aem.45.3.892-897.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M., Kolter R., Thomas C., Figurski D., Meyer R., Remaut E., Helinski D. R. Plasmid cloning vehicles derived from plasmids ColE1, F, R6K, and RK2. Methods Enzymol. 1979;68:268–280. doi: 10.1016/0076-6879(79)68019-9. [DOI] [PubMed] [Google Scholar]
- Klucas R. V., Hanus F. J., Russell S. A., Evans H. J. Nickel: A micronutrient element for hydrogen-dependent growth of Rhizobium japonicum and for expression of urease activity in soybean leaves. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2253–2257. doi: 10.1073/pnas.80.8.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasna A. I. Mutants of Escherichia coli with altered hydrogenase activity. J Gen Microbiol. 1984 Apr;130(4):779–787. doi: 10.1099/00221287-130-4-779. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambert G. R., Cantrell M. A., Hanus F. J., Russell S. A., Haddad K. R., Evans H. J. Intra- and interspecies transfer and expression of Rhizobium japonicum hydrogen uptake genes and autotrophic growth capability. Proc Natl Acad Sci U S A. 1985 May;82(10):3232–3236. doi: 10.1073/pnas.82.10.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepo J. E., Hanus F. J., Evans H. J. Chemoautotrophic growth of hydrogen-uptake-positive strains of Rhizobium japonicum. J Bacteriol. 1980 Feb;141(2):664–670. doi: 10.1128/jb.141.2.664-670.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macy J., Kulla H., Gottschalk G. H2-dependent anaerobic growth of Escherichia coli on L-malate: succinate formation. J Bacteriol. 1976 Feb;125(2):423–428. doi: 10.1128/jb.125.2.423-428.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R. J., Campbell N. E., Hanus F. J., Simpson F. B., Russell S. A., Evans H. J. Expression of hydrogenase activity in free-living Rhizobium japonicum. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3258–3262. doi: 10.1073/pnas.75.7.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- PECK H. D., Jr, GEST H. Formic dehydrogenase and the hydrogenlyase enzyme complex in coli-aerogenes bacteria. J Bacteriol. 1957 Jun;73(6):706–721. doi: 10.1128/jb.73.6.706-721.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STICKLAND L. H. The determination of small quantities of bacteria by means of the biuret reaction. J Gen Microbiol. 1951 Oct;5(4):698–703. doi: 10.1099/00221287-5-4-698. [DOI] [PubMed] [Google Scholar]
- Schneider K., Schlegel H. G. Purification and properties of soluble hydrogenase from Alcaligenes eutrophus H 16. Biochim Biophys Acta. 1976 Nov 8;452(1):66–80. doi: 10.1016/0005-2744(76)90058-9. [DOI] [PubMed] [Google Scholar]
- Stults L. W., O'Hara E. B., Maier R. J. Nickel is a component of hydrogenase in Rhizobium japonicum. J Bacteriol. 1984 Jul;159(1):153–158. doi: 10.1128/jb.159.1.153-158.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto I., Ishimoto M. Hydrogen-dependent growth of Escherichia coli in anaerobic respiration and the presence of hydrogenases with different functions. J Biochem. 1978 Sep;84(3):673–679. doi: 10.1093/oxfordjournals.jbchem.a132172. [DOI] [PubMed] [Google Scholar]