Abstract
Matrix metalloproteinase 13 (MMP13) is a key enzyme implicated in the degradation of the extracellular matrix in osteoarthritis. Clinical administration of broad spectrum MMP inhibitors such as marimastat has been implicated in severe musculo-skeletal side effects. Consequently, research has been focused on designing inhibitors that selectively inhibit MMP13, thereby circumventing musculo-skeletal toxicities. A series of pyrimidine dicarboxamides were recently shown to be highly selective inhibitors of MMP13 with a novel binding mode. We have applied a molecular ruler to this exosite by dual inhibition studies involving a potent dicarboxamide in the presence of two metal chelators of different sizes. A larger hydroxamate mimic overlaps and antagonizes binding of the dicarboxamide to the exosite whereas the much smaller acetohydroxamate synergizes with the dicarboxamide. These studies elucidate the steric requirement for compounds that fit exclusively into the active site, a mandate for generating highly selective MMP13 inhibitors.
Keywords: matrix metalloproteinase 13, pyrimidine dicarboxamides, hydroxamates, synergism, mutual exclusivity, inhibition
Articular cartilage consists of one cell type, the chondrocyte, which is embedded within the extracellular matrix (ECM) of predominantly type II collagen and aggrecan (a large aggregating proteoglycan). Type II collagen acts as a scaffold and confers the tensile strength of cartilage whereas aggrecan confers load bearing ability and compressibility. A healthy cartilage ECM is in a state of dynamic equilibrium with a fine balance between synthesis and degradation. In arthritis, this delicate balance is disrupted and ECM degradation occurs. The major proteases implicated in ECM degradation are aggrecanases and matrix metalloproteinases (MMPs), a family of 23 zinc endopeptidases that are classified according to the ECM components that they degrade. There are collagenases, gelatinases, stromelysins, membrane type (MT), and glycosylphosphatidylinositol-anchored enzymes (Greenwald et al. 1999; Mort and Billington 2001).
Degradation of the collagenous network is excessive in arthritis and may be mediated by the collagenolytic MMPs (1, 8, 13, and 14), which all cleave fibrillar or type II collagen into characteristic three-quarter and one-quarter length fragments (Chung et al. 2004). MMP13 has been a key target for osteoarthritis due to its enhanced mRNA levels (Neuhold et al. 2001) in the progression of disease, the fact that its preferred substrate is type II collagen (versus MMP-1 and -8), and also by the observation that natural and synthetic MMP13 inhibitors prevent interleukin induced cartilage destruction (Tardif et al. 2004).
As a result of intense study, much is known about the biochemistry of MMP13. MMP13 is synthesized as a proenzyme and must be processed by proteolytic cleavage at the N terminus to generate the active form. MMP13 has 471 amino acids and is composed of multiple domains: a hydrophobic signal peptide necessary for the secretion of the enzyme, a prodomain associated with latency, a catalytic domain containing the zinc binding site, and a hemopexin-like domain that is needed for collagenolytic activity (Overall 2002). In vivo, MMP-14 (MT1-MMP) and MMP2 are responsible for activation of the proenzyme. However, in vitro the enzyme can be activated using a 1 mM solution of p-aminophenyl mercuric acetate (Knauper et al. 1996).
The use of broad spectrum MMP inhibitors such as marimastat led to the observation of severe musculo-skeletal syndrome (Peterson 2006). Consequently, efforts were refocused on designing inhibitors that selectively inhibit MMP13. Engel et al. (2005) reported that a series of pyrimidine dicarboxamides inhibit MMP13 with high selectivity and that crystal structures of these compounds with the enzyme reveal a novel binding mode characterized by the absence of interactions between the inhibitors and the catalytic zinc, i.e., an exosite. The present work utilizes dual inhibition studies involving the most potent dicarboxamide {IC50 = 4–27 nM} (Fig. 1A) and differentially sized metal chelators (hydroxamate mimic {IC50 = 0.5–1.4 nM}, acetohydroxamate {mM efficacy}; Fig. 1, B and C, respectively) to allow for the construction of a molecular ruler for this exosite. These studies help us to understand the steric requirements for compounds that fit exclusively into the exosite, which may be necessary for generating highly selective MMP13 inhibitors. We have incorporated the use of a linear peptide substrate as well as a triple helical peptide (THP), which is more representative of the physiological collagen substrate.
Figure 1.
Structure of all three inhibitors discussed. (A) Pyrimidine dicarboxamide. (B) Hydroxamate mimic. (C) Acetohydroxamate.
Results and Discussion
The MMPs have received widespread attention over the past several decades as a tractable target for osteoarthritis. Many research groups have designed potent inhibitors that encompass a range of chemotypes such as hydroxamic acids, phosphinic acids (Makaritis et al. 2003), carboxylic acids (Levin et al. 2001), and conformationally constrained thiols (Freskos et al. 1999), all of which exert their inhibitory effect by chelating the catalytic zinc. However, these compounds all suffer from a lack of specificity toward MMP13, and there is compelling evidence to link this lack of specificity to the musculo-skeletal effects that plague MMP inhibitors in clinical trials (Hutchinson et al. 1998; Brown 2000; Bigg and Rowan 2001). Aventis reported a potent 72-nM compound (Engel et al. 2005) (pyrimidine dicarboxamide; Fig. 1A) that does not chelate the zinc ion but instead binds to a “specificity loop” (denoted by an asterisk in Fig. 2) in MMP13. This region of the protein structure exhibits diversity across the MMP family with differing amino acids. The crystallographic conformation of the MMP13 specificity loop is shown in Figure 2. In this conformation, side chain and backbone atoms of MMP13 make key interactions with the pyrimidine dicarboxamide inhibitor. We hypothesize that MMPs with different residue identities and sequence lengths are not able to assume a conformation of the specificity loop compatible with binding of this allosteric inhibitor, thereby resulting in selectivity for MMP13.
Figure 2.

(A) Overlaid structure of the catalytic domain of MMP13 with pyrimidine dicarboxamide (green) and hydroxamate mimic (magenta). The teal and gold ribbon diagrams represent MMP13 and exhibit a high degree of flexibility in the “selectivity loop” (denoted by *), which may be attributable to the presence of a glycine residue. The two inhibitors overlap by 6 Å. (B) Docked structure of MMP 13 with pyrimidine dicarboxamide (green) and acetohydroxamate (orange). These two docked structures are 6 Å apart.
In order to further elucidate the steric requirements for binding to this “exosite” region, a study was undertaken whereby the effect of the pyrimidine dicarboxamide was examined in concert with either acetohydroxamate or a larger hydroxamate mimic. However, before this could be accomplished, two substrates, a linear and a triple-helical peptide, were characterized using full-length MMP13. The Km values were determined as 1.5 ± 0.18 μM and 35 ± 1.6 μM respectively, consistent with the literature (Lauer-Fields et al. 2001). All subsequent single or dual inhibition studies incorporated concentrations that were as close to the Km as possible ([linear peptide] = 2 μM and the [THP] = 25 μM).
The overlaid structures of hydroxamate mimic (magenta) and pyrimidine dicarboxamide (green; Fig. 2A) in the catalytic domain of MMP13 show that there is a 6 Å overlap between the two structures. The structural model indicates that each compound would interfere with the other's ability to bind to their respective sites. Dual inhibition studies using a Yonetani-Theorell analysis would therefore predict antagonistic binding of the dicarboxamide in the presence of the zinc chelator. In contrast, the docked MMP13 structure containing acetohydroxamate (orange) and pyrimidine dicarboxamide (green) (Fig. 2B) shows that both inhibitors bind within the zinc region and the exosite region, respectively, separated by 6 Å. Dual inhibition studies would therefore predict synergistic binding between these two inhibitors.
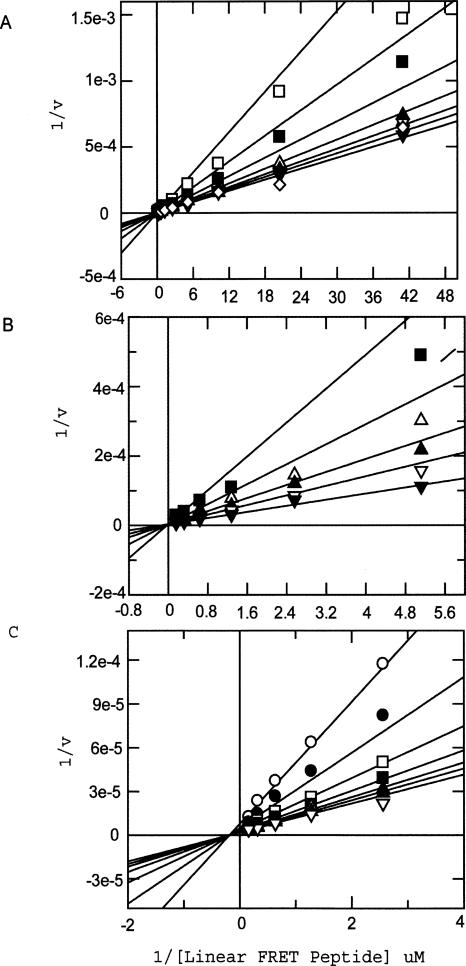
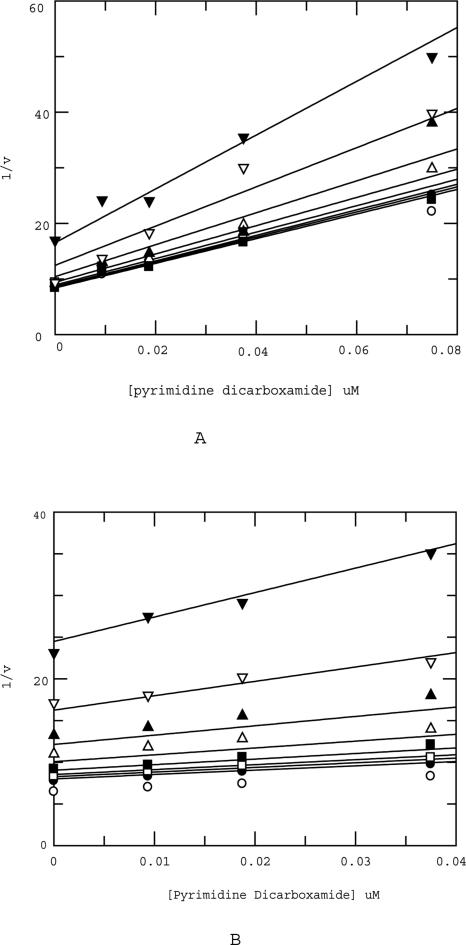
The mode of action of all three inhibitors was confirmed prior to dual inhibition studies. As expected, the acetohydroxamate and the hydroxamate mimic both bind competitively with respect to the peptide substrate as evidenced by the fit of the data to a competitive model (Fig. 3, A and B, respectively). However, the pyrimidine dicarboxamide binds in a noncompetitive manner to MMP13 (Fig. 3C), consistent with the crystal structure showing binding to an exosite. Dual inhibition studies were conducted in which dicarboxamide was titrated against fixed and varying concentrations of hydroxamate mimic. These studies, also known as mutual exclusivity studies, can predict the binding cooperativity between two inhibitors. The experimental data obtained from the studies were fit globally to the equation of Yonetani-Theorell using Grafit. The term B in the Yonetani-Theorell equation is a constant that defines the degree of interaction between the two inhibitors. If the two inhibitors bind to the enzyme in a mutually exclusive manner, the value of B is infinite. When the two inhibitors are not mutually exclusive, B ≠ ∞. B < 1 is indicative of positive cooperativity in the binding of the two inhibitors and 1 < B < ∞ signals antagonism in the binding of the two inhibitors (Yonetani and Theorell 1964). A fit of the data to the Yonetani-Theorell equation revealed that the B value is infinite for hydroxamate mimic and dicarboxamide consistent with a model (Fig. 2A) in which the hydroxamate mimic antagonizes binding of the exosite inhibitor (Fig. 4A,B). This is in agreement with the predictions made from docking the inhibitors in the catalytic domain of MMP13. The similarity of results between the linear and THP substrate suggests that there are no additional exosite regions involved in the binding of the pyrimidine dicarboxamide when a more physiologically relevant substrate is used. Although THP substrates represent greatly simplified collagen models (Lauer-Fields et al. 2001; Minond et al. 2006), based on the similarity of results between linear and THP substrate it is highly unlikely that the inhibitors would behave differently with a native collagen substrate. Dual inhibition studies of dicarboxamide with a much smaller zinc binder, acetohydroxamate (AHA), yields dramatically different results. Once again, dicarboxamide was titrated against fixed and varying concentrations of acetohydroxamate. However, the interaction term B < 1, which is consistent with a model in which acetohydroxamate synergizes with the dicarboxamide (Fig. 5A,B). This is in agreement with predictions made from docking both inhibitors simultaneously into the catalytic domain of MMP13 (Fig. 2B). Furthermore, there were no detectable differences when either linear or THP substrates were used. This result demonstrates that there are very strict steric requirements, a 6 Å distance, for “confining” the inhibitor to the exosite region. In this study 6 Å from the zinc catalytic site is sufficient to confine the dicarboxamide to the exosite. A more precise molecular ruler can be constructed by synthesizing incrementally sized hydroxamates and profiling their effect on the dicarboxamide. By combining a properly sized hydroxamate “fragment” with the dicarboxamide, one could envision an even more potent, selective MMP13 inhibitor (Hajduk and Greer 2007).
Figure 3.
(A) Acetohydroxamate fits best to a competitive model described in Materials and Methods. The same fit was also obtained (data not shown) when the THP substrate was used in lieu of the linear substrate. Legend (varying concentrations of inhibitor): □ 20 mM, ■ 10 mM, △ 5 mM, ▲ 2.5 mM, ◇ 1.25 mM, ▼ 0 mM. (B) Hydroxamate mimic fit best to a competitive model described in Materials and Methods. The same fit was also obtained (data not shown) when the THP substrate was used in lieu of the linear substrate. Legend (varying concentrations of inhibitor): ■ 2.5 nM, △ 1.25 nM, ▲ 0.625 nM, ▽ 0.3125 nM, ▼ 0 nM. (C) Pyrimdine dicarboxamide fit best to a noncompetitive model described in Materials and Methods. The same fit was also obtained (data not shown) when the THP substrate was used in lieu of the linear substrate. Legend (varying concentrations of inhibitor): ○ 125 nM, ● 62.5 nM, □ 31.25 nM, ■ 15.625 nM, △ 7.8125 nM, ▲ 3.90625 nM, ▽ 0 nM.
Figure 4.
(A) Yonetani-Theorell analysis of pyrimidine dicarboxamide in the presence of hydroxamate mimic (linear substrate). Legend (varying concentrations of hydroxamate mimic): ○ 0 nM, ● 0.125 nM, □ 0.25 nM, ■ 0.5 nM, △ 0.75 nM, ▲ 1.25 nM, ▽ 2.5 nM. The B value from the fit was >>>1, indicating antagonism of binding of pyrimidine dicarboxamide by the hydroxamate mimic. (B) Yonetani-Theorell analysis of pyrimidine dicarboxamide in the presence of hydroxamate mimic (THP substrate). Legend (varying concentrations of hydroxamate mimic): ○ 0 nM, □ 0.25 nM, ■ 0.5 nM, △ 0.75 nM, ▲ 1.25 nM, ▽ 2.5 nM, ▼ 5 nM. The B value from the fit was >>>1, indicating antagonism of binding of pyrimidine dicarboxamide by the hydroxamate mimic.
Figure 5.
(A) Yonetani-Theorell analysis of pyrimidine dicarboxamide in the presence of acetohydroxamate (linear substrate). Legend (varying concentrations of acetohydroxamate): ○ 0 μM, ● 156.25 μM, □ 312.5 μM, ■ 625 μM, △ 1250 μM, ▲ 2500 μM, ▽ 5000 μM, ▼ 10,000 μM. The B value from the fit was <1, indicating synergism of binding of pyrimidine dicarboxamide by the acetohydroxamate. (B) Yonetani-Theorell analysis of pyrimidine dicarboxamide in the presence of acetohydroxamate (THP substrate). Legend (varying concentrations of acetohydroxamate): ○ 0 μM, ● 156.25 μM, □ 312.5 μM, ■ 625 μM, △ 1250 μM, ▲ 2500 μM, ▽ 5000 μM, ▼ 10,000 μM. The B value from the fit was <1, indicating synergism of binding of pyrimidine dicarboxamide by the acetohydroxamate.
In conclusion, we used three different classes of MMP13 inhibitors to probe the sterics of a key exosite in MMP13. The results from the mutual exclusivity studies show that the exosite specific binder dicarboxamide has synergy with the acetohydroxamate in MMP13 binding but binds mutually exclusive with the hydroxamate mimic. These results provide useful information of the sterics of the exosite, an understanding that could lead to the design of selective MMP13 inhibitors that can diminish the clinical liabilities associated with nonspecific MMP13 inhibitors.
Materials and Methods
Activation of pro-MMP13
The proenzyme (CC1047, Chemicon) was activated according to the manufacturer's instructions. We mixed 19.5 μL of CC1047 with 0.5 μL of 40 mM APMA (p-aminophenyl mercuric acetate, Calbiochem 164610, 40 mM stock made up in 100% DMSO) and incubated the mixture for 30 min at 37°C. The activated enzyme was then placed on ice before use in subsequent assays. A time course of activation (data not shown) confirmed that the enzyme was fully activated within 30 min. The enzyme was not kept on ice for longer than 2 h.
MMP13 assays
A 40-μL assay was set up in a 96-well black costar round bottom polystyrene plate (costar #3792). In dual inhibition studies both inhibitors (sequential 10-μL additions) were added first followed by 10 μL of enzyme (final concentration of active enzyme is 0.4 nM when linear substrate is used and 4 nM when THP substrate is used). The reaction was then initiated by addition of 10 μL substrate such that the final concentration of the fluorescent substrate is 2 μM and the final THP substrate is 25 μM. In all assays, the final DMSO concentration did not exceed 5%. All reactions were monitored on an Envision plate reader with settings of 485 nm excitation, 535 nm emission (linear substrate) and 320 nm excitation, 430 nm emission, 385 dichroic mirror (THP substrate). Only rates from linear time courses were used to assess kinetic parameters. Enzyme and substrate stocks were diluted using 1× assay buffer (linear peptide assay: 1× = 50 mM HEPES pH 7.5, 10 mM CaCl2, 200 mM NaCl, and 0.0625% CHAPS; THP assay: 1× = 50 mM HEPES pH 7.5, 10 mM CaCl2, 200 mM NaCl, and 0.15% CHAPS).
Yonetani-Theorell analysis
In this analysis, the following equation was used:
 |
where vij is the initial velocity in the presence of both inhibitors, Ki and Kj are the dissociation constants for the inhibitors I and J, respectively, and B is the interaction term that defines the effect of the binding of one inhibitor on the affinity of the second inhibitor. In all dual inhibition studies, the putative exosite compound N,N′-bis[(3-methlyphenyl)methyl]-4,6-pyrimidinedicarboxamide was varied from 0 to 0.5 μM, the active site inhibitor (2R,3R)-1-[(4-{[2-chloro-4-fluorphenyl)methyl]oxy}phenyl)sulfonyl]-N,3-dihydroxy-3-methly-2-piperidinecarboxamide was varied from 0 to 5 nM, and the acetohydroxamate was varied from 0 to 10 mM.
Km determination
The Km was determined by fitting the data to the Michaelis-Menten equation:
 |
where S is the substrate concentration and v is the measured rate of the reaction.
Mode of inhibition
The equations for fitting to the various models are as follows:
MMP13 substrates
The fluorescent substrate used had the following sequence: (5-FAM-TPGPLGL[Dap(DNP)]ARRK)5-TAMRA-amide (where FAM is 6-carboxyfluorescein, Dap is 2,3-diaminopropionic acid, DNP is 2,4-dinitrophenyl, and TAMRA is tetramethyl-6-carboxyrhodamine) and was provided by 21st Century Biochemicals. The purity was assessed to be 90.36% by HPLC-MS. Stocks of this solution were made by dissolving it in 1× assay buffer as described above supplemented with 50% methanol and 2% DMSO. The concentration was calculated using a UV-VIS Cary Spectrophotometer by performing a 1:10-fold dilution of stocks into blank 1× assay buffer. The concentrations were determined by Beer's Law's plot:
where ε is the extinction coefficient of the fluorophore in Lmol−1cm−1 (20,960 Lmol−1cm−1 for FAM and 29,100 Lmol−1cm−1 for TAMRA), b is the path length (1 cm), and c is the molar concentration of the agent. Stock concentrations were calculated using the ε of FAM. The stocks were stored at −20°C and were only opened briefly on ice before making up intermediate substrate stocks in 1× assay buffer. This treatment prevents evaporation of methanol, but periodically the concentration of the stock was measured to correct for evaporation.
The triple-helical substrate fTHP-4 had the sequence (Gly-Pro-Hyp)5-Gly-Pro-Lys(MCa)-Gly-Pro-Gln-Gly-Leu-Arg-Gly-Gln-Lys(Dnp)-Gly-Val-Arg-(Gly-Pro-Hyp)5NH2.. This substrate, which was synthesized as described (Lauer-Fields et al. 2001), was dissolved in 100% DMSO to make up 5 mM storage stocks.
Inhibitor synthesis: Preparation of N,N′-bis[(3-methylphenyl)methyl]-4,6-pyrimidinedicarboxamide
We added 200 μL of 3-methylbenzylamine to a 0.5-mL microwave-compatible reaction vessel containing 30 mg (150 μM) of dimethyl 4,6-pyrimidinedicarboxylate. The neat mixture was subjected to heating via microwave irradiation at 200°C for 10 min. Upon cooling, the reaction mixture was evaporated under a stream of nitrogen and the residue subjected to HPLC purification to yield 35 mg (63%) of white solid (LCMS with{M + H} = 375.1).
Modeling studies
The publicly available crystal structures of MMP13 containing nonmetal binding dicarboxamide [1XUC] (Engel et al. 2005) and metal-chelating compound containing a hydroxamate similar to the described hydroxamate mimic [2D1N] (Kohno et al. 2006) were downloaded from the PDB. 2D1N was used as a model binding site for docking metal-chelating hydroxamate mimic using Flo (PyMOL, DeLano Scientific). The resulting optimized docked pose of hydroxamate mimic was overlaid with 1XUC in MOE (http://www.chemcomp.com). The overlay of the nonmetal binding 1XUC (green) and docked model of hydroxamate (magenta) is shown in Figure 2A.
Cocrystal structure 1XUC was used for docking acetohydroxamate. Flo was used to dock acetohydroxamate to MMP13 in the presence of dicarboxamide. An optimized docking model reveals acetohydroxamate chelating tightly to the MMP13 catalytic zinc with a 6 Å distance to dicarboxamide (Fig. 2B).
Acknowledgments
We gratefully acknowledge partial support of this work from the National Institutes of Health (MH 078948 and CA 98799 to G.B.F.). All figures were made using PyMOL (http://www.pymol.org).
Footnotes
Reprint requests to: Lata T. Gooljarsingh, GlaxoSmithKline Pharmaceuticals, 500 Arcola Road, Collegeville, PA 19426, USA; e-mail: gooljat@wyeth.com; fax: (484) 865-4315.
Article published online ahead of print. Article and publication date are at http://www.proteinscience.org/cgi/doi/10.1110/ps.073130208.
References
- Bigg, H.F., Rowan, A.D. The inhibition of metalloproteinases as a therapeutic target in rheumatoid arthritis and osteoarthritis. Curr. Opin. Pharmacol. 2001;1:314–320. doi: 10.1016/s1471-4892(01)00055-8. [DOI] [PubMed] [Google Scholar]
- Brown, P.D. Ongoing trials with matrix metalloproteinase inhibitors. Expert Opin. Investig. Drugs. 2000;9:2167–2177. doi: 10.1517/13543784.9.9.2167. [DOI] [PubMed] [Google Scholar]
- Chung, L., Dinakarpandian, D., Yoshida, N., Lauer-Fields, J.L., Fields, G.B., Visse, R., Nagase, H. Collagenase unwinds triple-helical collagen prior to peptide bond hydrolysis. EMBO J. 2004;23:3020–3030. doi: 10.1038/sj.emboj.7600318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel, C.K., Pirard, B., Schimanski, S., Kirsch, R., Habermann, J., Klinger, O., Schlotte, V., Weithmann, K.U., Wendt, K.U. Structural basis for the highly selective inhibition of MMP13. Chem. Biol. 2005;12:181–189. doi: 10.1016/j.chembiol.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Freskos, J.N., McDonald, J.J., Mischke, B.V., Mullins, P.B., Shieh, H.S., Stegeman, R.A., Stevens, A.M. Synthesis and identification of conformationally constrained selective MMP inhibitors. Bioorg. Med. Chem. Lett. 1999;9:1757–1760. doi: 10.1016/s0960-894x(99)00285-1. [DOI] [PubMed] [Google Scholar]
- Greenwald, R.A., Zucker, S., Golub, L.M. Inhibition of matrix metalloproteinases: Therapeutic applications. New York Academy of Sciences; New York: 1999. [Google Scholar]
- Hajduk, P.J., Greer, J. A decade of fragment-based drug design: Strategic advances and lessons learned. Nat. Rev. Drug Discov. 2007;6:211–219. doi: 10.1038/nrd2220. [DOI] [PubMed] [Google Scholar]
- Hutchinson, J.W., Tierney, G.M., Parsons, S.L., Davis, T.R.C. Dupuytren's disease and frozen shoulder induced by treatment with a matrix metalloproteinase inhibitor. J. Bone Joint Surg. Br. 1998;80-B:907–908. doi: 10.1302/0301-620x.80b5.8464. [DOI] [PubMed] [Google Scholar]
- Knauper, V., Will, H., Loez-Otin, C., Smith, B., Atkinson, S.J., Stanton, H., Hembry, R.M., Murphy, G. Cellular mechanisms for human procollagenase-3 (MMP13) activation. J. Biol. Chem. 1996;271:17124–17131. doi: 10.1074/jbc.271.29.17124. [DOI] [PubMed] [Google Scholar]
- Kohno, T., Hochigai, H., Yamashita, E., Tsukihara, T., Kanaoka, M. Crystal structures of the catalytic domain of human stromelysin-1 (MMP-3) and collagenase-3 (MMP13) with a hydroxamic acid inhibitor SM-25453. Biochem. Biophys. Res. Commun. 2006;344:315–322. doi: 10.1016/j.bbrc.2006.03.098. [DOI] [PubMed] [Google Scholar]
- Lauer-Fields, J.L., Broder, T., Sritharan, T., Chung, L., Nagase, H., Fields, G.B. Kinetic analysis of matrix metalloproteinase activity using fluorogenic triple-helical substrates. Biochemistry. 2001;40:5795–5803. doi: 10.1021/bi0101190. [DOI] [PubMed] [Google Scholar]
- Levin, J.I., Chen, J., Du, M., Hogan, M., Kincaid, S., Nelson, F.C., Venkatesan, A.M., Wehr, T., Zask, A., DiJoseph, J., et al. The discovery of anthranilic acid-based MMP inhibitors. Part 2: SAR of the 5-position and P1(1) groups. Bioorg. Med. Chem. Lett. 2001;11:2189–2192. doi: 10.1016/s0960-894x(01)00419-x. [DOI] [PubMed] [Google Scholar]
- Makaritis, A., Georgiadis, D., Dive, V., Yiotakis, A. Diastereoselective solution and multipin-based combinatorial array synthesis of a novel class of potent phosphinic metalloprotease inhibitors. Chemistry (Easton) 2003;9:2079–2094. doi: 10.1002/chem.200204456. [DOI] [PubMed] [Google Scholar]
- Minond, D., Lauer-Fields, J.L., Cudic, M., Overall, C.M., Pei, D., Brew, K., Visse, R., Nagase, H., Fields, G.B. The roles of substrate thermal stability and P2 and P1′ subsite identity on matrix metalloproteinase triple-helical peptidase activity and collagen specificity. J. Biol. Chem. 2006;281:38302–38313. doi: 10.1074/jbc.M606004200. [DOI] [PubMed] [Google Scholar]
- Mort, J.S., Billington, C.J. Articular cartilage and changes in arthritis matrix degradation. Arthritis Res. 2001;3:3370–3374. doi: 10.1186/ar325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhold, L.A., Killar, L., Zhao, W., Sung, M.L., Warner, L., Kulik, J. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J. Clin. Invest. 2001;107:35–44. doi: 10.1172/JCI10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall, C.M. Molecular determinants of metalloproteinase substrate specificity. Mol. Biotechnol. 2002;22:51–86. doi: 10.1385/MB:22:1:051. [DOI] [PubMed] [Google Scholar]
- Peterson, J.T. The importance of estimating the therapeutic index in the development of matrix metalloproteinase inhibitors. Cardiovasc. Res. 2006;69:677–687. doi: 10.1016/j.cardiores.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Tardif, G., Reboul, P., Pelletier, J.P., Martel-Pelletier, J. Ten years in the life of an enzyme: The story of the human MMP-13(collagenase) Mod. Rheumatol. 2004;14:197–204. doi: 10.1007/s10165-004-0292-7. [DOI] [PubMed] [Google Scholar]
- Yonetani, T., Theorell, H. Studies on liver alcohol hydrogenase complexes III. Multiple inhibition kinetics in the presence of two competitive inhibitors. Arch. Biochem. Biophys. 1964;106:243–251. doi: 10.1016/0003-9861(64)90184-5. [DOI] [PubMed] [Google Scholar]