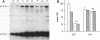Abstract
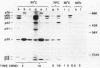
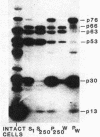
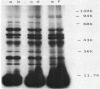
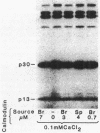
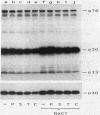
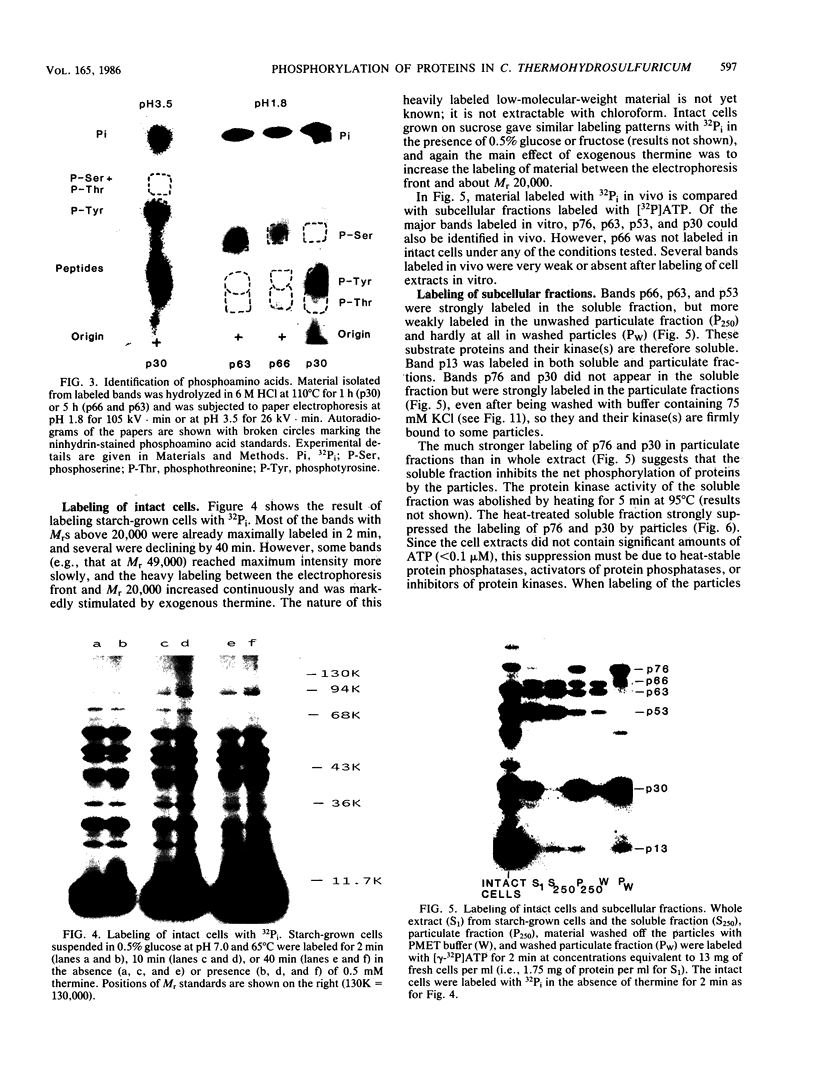
Cell extracts of the thermophile Clostridium thermohydrosulfuricum catalyzed the phosphorylation by [gamma-32P]ATP of several endogenous proteins with Mrs between 13,000 and 100,000. Serine and tyrosine were the main acceptors. Distinct substrate proteins were found in the soluble (e.g., proteins p66, p63, and p53 of Mrs 66,000, 63,000, and 53,000, respectively) and particulate (p76 and p30) fractions, both of which contained protein kinase and phosphatase activity. The soluble fraction suppressed the phosphorylation of particulate proteins and contained a protein kinase inhibitor. Phosphorylation of p53 was promoted by 10 microM fructose 1,6-bisphosphate or glucose 1,6-bisphosphate and suppressed by hexose monophosphates, whereas p30 and p13 were suppressed by 5 microM brain (but not spinach) calmodulin. Polyamines, including the "odd" polyamines characteristic of thermophiles, modulated the labeling of most of the phosphoproteins. Apart from p66, all the proteins labeled in vitro were also rapidly labeled in intact cells by 32Pi. Several proteins strongly labeled in vivo were labeled slowly or not at all in vitro.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cheng Y. S., Chen L. B. Detection of phosphotyrosine-containing 34,000-dalton protein in the framework of cells transformed with Rous sarcoma virus. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2388–2392. doi: 10.1073/pnas.78.4.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Enami M., Ishihama A. Protein phosphorylation in Escherichia coli and purification of a protein kinase. J Biol Chem. 1984 Jan 10;259(1):526–533. [PubMed] [Google Scholar]
- Feuerstein N., Cooper H. L. Rapid protein phosphorylation induced by phorbol ester in HL-60 cells. Unique alkali-stable phosphorylation of a 17,000-dalton protein detected by two-dimensional gel electrophoresis. J Biol Chem. 1983 Sep 10;258(17):10786–10793. [PubMed] [Google Scholar]
- Garvie E. I. Bacterial lactate dehydrogenases. Microbiol Rev. 1980 Mar;44(1):106–139. doi: 10.1128/mr.44.1.106-139.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Tyrosine protein kinases and their substrates: an overview. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:443–455. [PubMed] [Google Scholar]
- Iwasa Y., Yonemitsu K., Matsui K., Fukunaga K., Miyamoto E. Calmodulin-like activity in the soluble fraction of Escherichia coli. Biochem Biophys Res Commun. 1981 Feb 12;98(3):656–660. doi: 10.1016/0006-291x(81)91164-5. [DOI] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lukas T. J., Iverson D. B., Schleicher M., Watterson D. M. Structural characterization of a higher plant calmodulin : spinacia oleracea. Plant Physiol. 1984 Jul;75(3):788–795. doi: 10.1104/pp.75.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manai M., Cozzone A. J. Analysis of the protein-kinase activity of Escherichia coli cells. Biochem Biophys Res Commun. 1979 Dec 14;91(3):819–826. doi: 10.1016/0006-291x(79)91953-3. [DOI] [PubMed] [Google Scholar]
- Mäenpä P. H. Effects of polyamines and polyanions on a cyclic nucleotide-independent and a cyclic AMP-dependent protein kinase. Biochim Biophys Acta. 1977 Jul 21;498(1):294–305. doi: 10.1016/0304-4165(77)90267-7. [DOI] [PubMed] [Google Scholar]
- Ono-Iwashita Y., Oshima T., Imahori K. In vitro protein synthesis at elevated temperature by an extract of an extreme thermophile. Effects of polyamines on the polyuridylic acid-directed reaction. Arch Biochem Biophys. 1975 Dec;171(2):490–499. doi: 10.1016/0003-9861(75)90058-2. [DOI] [PubMed] [Google Scholar]
- Oshima T. A pentaamine is present in an extreme thermophile. J Biol Chem. 1982 Sep 10;257(17):9913–9914. [PubMed] [Google Scholar]
- Oshima T. Thermine: a new polyamine from an extreme thermophile. Biochem Biophys Res Commun. 1975 Apr 21;63(4):1093–1098. doi: 10.1016/0006-291x(75)90681-6. [DOI] [PubMed] [Google Scholar]
- Schrama L. H., Weeda G., Edwards P. M., Oestreicher A. B., Schotman P. Multiple phosphorylation of pp30, a rat brain polyribosomal protein, sensitive to polyamines and corticotropin. Biochem J. 1984 Dec 15;224(3):747–753. doi: 10.1042/bj2240747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Y., Koshland D. E., Jr Evidence for protein kinase activities in the prokaryote Salmonella typhimurium. J Biol Chem. 1978 Nov 10;253(21):7605–7608. [PubMed] [Google Scholar]
- Wool I. G. The structure and function of eukaryotic ribosomes. Annu Rev Biochem. 1979;48:719–754. doi: 10.1146/annurev.bi.48.070179.003443. [DOI] [PubMed] [Google Scholar]