Abstract
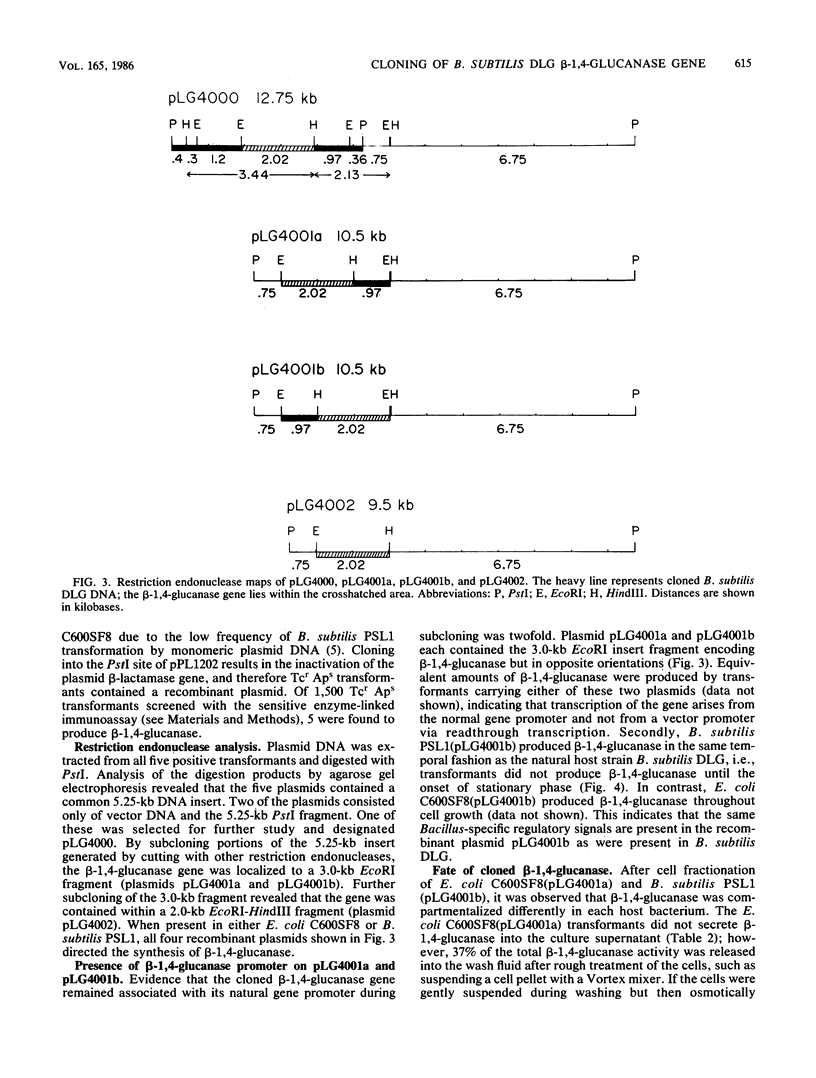
The gene encoding beta-1,4-glucanase in Bacillus subtilis DLG was cloned into both Escherichia coli C600SF8 and B. subtilis PSL1, which does not naturally produce beta-1,4-glucanase, with the shuttle vector pPL1202. This enzyme is capable of degrading both carboxymethyl cellulose and trinitrophenyl carboxymethyl cellulose, but not more crystalline cellulosic substrates (L. M. Robson and G. H. Chambliss, Appl. Environ. Microbiol. 47:1039-1046, 1984). The beta-1,4-glucanase gene was localized to a 2-kilobase (kb) EcoRI-HindIII fragment contained within a 3-kb EcoRI chromosomal DNA fragment of B. subtilis DLG. Recombinant plasmids pLG4000, pLG4001a, pLG4001b, and pLG4002, carrying this 2-kb DNA fragment, were stably maintained in both hosts, and the beta-1,4-glucanase gene was expressed in both. The 3-kb EcoRI fragment apparently contained the beta-1,4-glucanase gene promoter, since transformed strains of B. subtilis PSL1 produced the enzyme in the same temporal fashion as the natural host B. subtilis DLG. B. subtilis DLG produced a 35,200-dalton exocellular beta-1,4-glucanase; intracellular beta-1,4-glucanase was undetectable. E. coli C600SF8 transformants carrying any of the four recombinant plasmids produced two active forms of beta-1,4-glucanase, an intracellular form (51,000 +/- 900 daltons) and a cell-associated form (39,000 +/- 400 daltons). Free exocellular enzyme was negligible. In contrast, B. subtilis PSL1 transformed with recombinant plasmid pLG4001b produced three distinct sizes of active exocellular beta-1,4-glucanase: approximately 36,000, approximately 35,200, and approximately 33,500 daltons. Additionally, B. subtilis PSL1(pLG4001b) transformants contained a small amount (5% or less) of active intracellular beta-1,4-glucanase of three distinct sizes: approximately 50,500, approximately 38,500 and approximately 36,000 daltons. The largest form of beta-1,4-glucanase seen in both transformants may be the primary, unprocessed translation product of the gene.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan R. J., Mendelson N. H., Brooks D., Young F. E. Regulation of the bacterial cell wall: analysis of a mutant of Bacillus subtilis defective in biosynthesis of teichoic acid. J Bacteriol. 1972 Apr;110(1):281–290. doi: 10.1128/jb.110.1.281-290.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béguin P. Detection of cellulase activity in polyacrylamide gels using Congo red-stained agar replicas. Anal Biochem. 1983 Jun;131(2):333–336. doi: 10.1016/0003-2697(83)90178-1. [DOI] [PubMed] [Google Scholar]
- Canosi U., Morelli G., Trautner T. A. The relationship between molecular structure and transformation efficiency of some S. aureus plasmids isolated from B. subtilis. Mol Gen Genet. 1978 Nov 9;166(3):259–267. doi: 10.1007/BF00267617. [DOI] [PubMed] [Google Scholar]
- Cornelis P., Digneffe C., Willemot K. Cloning and expression of a Bacillus coagulans amylase gene in Escherichia coli. Mol Gen Genet. 1982;186(4):507–511. doi: 10.1007/BF00337957. [DOI] [PubMed] [Google Scholar]
- Henkin T. M., Chambliss G. H. Genetic mapping of a mutation causing an alteration in Bacillus subtilis ribosomal protein S4. Mol Gen Genet. 1984;193(2):364–369. doi: 10.1007/BF00330694. [DOI] [PubMed] [Google Scholar]
- Hinchliffe E. Cloning and expression of a Bacillus subtilis Endo-1,3-1,4-beta-D-glucanase gene in Escherichia coli K12. J Gen Microbiol. 1984 May;130(5):1285–1291. doi: 10.1099/00221287-130-5-1285. [DOI] [PubMed] [Google Scholar]
- Huang J. S., Tang J. Sensitive assay for cellulase and dextranase. Anal Biochem. 1976 Jun;73(2):369–377. doi: 10.1016/0003-2697(76)90182-2. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MALAMY M., HORECKER B. L. The localization of alkaline phosphatase in E. coli K12. Biochem Biophys Res Commun. 1961 Jun 2;5:104–108. doi: 10.1016/0006-291x(61)90020-1. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Ostroff G. R., Pène J. J. Molecular cloning with bifunctional plasmid vectors in Bacillus subtilis. II. Transfer of sequences propagated in Escherichia coli to B. subtilis. Mol Gen Genet. 1984;193(2):306–311. doi: 10.1007/BF00330685. [DOI] [PubMed] [Google Scholar]
- Ostroff G. R., Pène J. J. Molecular cloning with bifunctional plasmid vectors in Bacillus subtilis: isolation of a spontaneous mutant of Bacillus subtilis with enhanced transformability for Escherichia coli-propagated chimeric plasmid DNA. J Bacteriol. 1983 Nov;156(2):934–936. doi: 10.1128/jb.156.2.934-936.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva I., Pettersson R. F., Kalkkinen N., Lehtovaara P., Sarvas M., Söderlund H., Takkinen K., Käriäinen L. Nucleotide sequence of the promoter and NH2-terminal signal peptide region of the alpha-amylase gene from Bacillus amyloliquefaciens. Gene. 1981 Oct;15(1):43–51. doi: 10.1016/0378-1119(81)90103-7. [DOI] [PubMed] [Google Scholar]
- Priest F. G. Extracellular enzyme synthesis in the genus Bacillus. Bacteriol Rev. 1977 Sep;41(3):711–753. doi: 10.1128/br.41.3.711-753.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson L. M., Chambliss G. H. Characterization of the cellulolytic activity of a Bacillus isolate. Appl Environ Microbiol. 1984 May;47(5):1039–1046. doi: 10.1128/aem.47.5.1039-1046.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Sashihara N., Kudo T., Horikoshi K. Molecular cloning and expression of cellulase genes of alkalophilic Bacillus sp. strain N-4 in Escherichia coli. J Bacteriol. 1984 May;158(2):503–506. doi: 10.1128/jb.158.2.503-506.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M. A., Ortlepp S. A., Ollington J. F., McConnell D. J. Nucleotide sequence of the 5' region of the Bacillus licheniformis alpha-amylase gene: comparison with the B. amyloliquefaciens gene. J Bacteriol. 1984 Apr;158(1):369–372. doi: 10.1128/jb.158.1.369-372.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K., Cameron J. R., Davis R. W. Functional genetic expression of eukaryotic DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1976 May;73(5):1471–1475. doi: 10.1073/pnas.73.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasantha N., Thompson L. D., Rhodes C., Banner C., Nagle J., Filpula D. Genes for alkaline protease and neutral protease from Bacillus amyloliquefaciens contain a large open reading frame between the regions coding for signal sequence and mature protein. J Bacteriol. 1984 Sep;159(3):811–819. doi: 10.1128/jb.159.3.811-819.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. L., Price C. W., Goldfarb D. S., Doi R. H. The subtilisin E gene of Bacillus subtilis is transcribed from a sigma 37 promoter in vivo. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1184–1188. doi: 10.1073/pnas.81.4.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]