Abstract
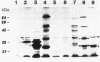
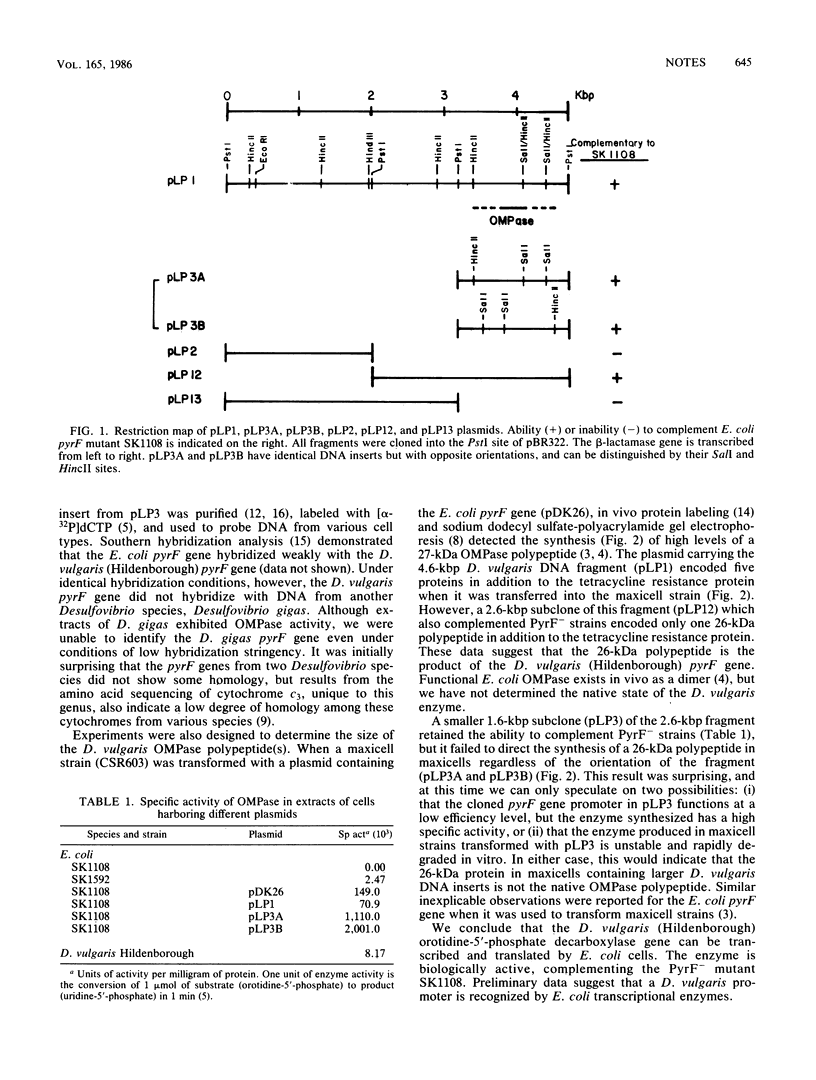
A PyrF- mutant of Escherichia coli (SK1108, pyrF::Tn5 Kanr) was complemented with the Desulfovibrio vulgaris (Hildenborough) structural gene for orotidine-5'-phosphate decarboxylase (EC 4.1.1.23). Either orientation of a 1.6-kilobase-pair D. vulgaris DNA fragment (pLP3B or pLP3A) complemented the PyrF- strain suggesting that the D. vulgaris pyrF promoter was functional. The apparent product of the D. vulgaris pyrF gene was a single 26-kilodalton polypeptide. These results demonstrate the utility of E. coli cloning systems in studying metabolic and energetic pathways in sulfate-reducing bacteria.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazaral M., Helinski D. R. Circular DNA forms of colicinogenic factors E1, E2 and E3 from Escherichia coli. J Mol Biol. 1968 Sep 14;36(2):185–194. doi: 10.1016/0022-2836(68)90374-4. [DOI] [PubMed] [Google Scholar]
- Donovan W. P., Kushner S. R. Cloning and physical analysis of the pyrF gene (coding for orotidine-5'-phosphate decarboxylase) from Escherichia coli K-12. Gene. 1983 Nov;25(1):39–48. doi: 10.1016/0378-1119(83)90165-8. [DOI] [PubMed] [Google Scholar]
- Donovan W. P., Kushner S. R. Purification and characterization of orotidine-5'-phosphate decarboxylase from Escherichia coli K-12. J Bacteriol. 1983 Nov;156(2):620–624. doi: 10.1128/jb.156.2.620-624.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Kellogg W. W., Cadle R. D., Allen E. R., Lazrus A. L., Martell E. A. The sulfur cycle. Science. 1972 Feb 11;175(4022):587–596. doi: 10.1126/science.175.4022.587. [DOI] [PubMed] [Google Scholar]
- Kushner S. R. In vivo studies of temperature-sensitive recB and recC mutants. J Bacteriol. 1974 Dec;120(3):1213–1218. doi: 10.1128/jb.120.3.1213-1218.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEGALL J., MAZZA G., DRAGONI N. LE CYTOCHROME C3 DE DESULFOVIBRIO GIGAS. Biochim Biophys Acta. 1965 May 18;99:385–387. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Peck H. D., Jr, LeGall J. Biochemistry of dissimilatory sulphate reduction. Philos Trans R Soc Lond B Biol Sci. 1982 Sep 13;298(1093):443–466. doi: 10.1098/rstb.1982.0091. [DOI] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voordouw G., Walker J. E., Brenner S. Cloning of the gene encoding the hydrogenase from Desulfovibrio vulgaris (Hildenborough) and determination of the NH2-terminal sequence. Eur J Biochem. 1985 May 2;148(3):509–514. doi: 10.1111/j.1432-1033.1985.tb08868.x. [DOI] [PubMed] [Google Scholar]