Abstract
Furrow ingression in animal cell cytokinesis is controlled by phosphorylation of myosin II regulatory light chain (mRLC). In C. elegans embryos, Rho-dependent Kinase (RhoK) is involved in, but not absolutely required for, this phosphorylation. The calmodulin effector Myosin Light Chain Kinase (MLCK) can also phosphorylate mRLC and is widely regarded as a candidate for redundant function with RhoK. However, our results show that RNAi against C. elegans calmodulin and candidate MLCKs had no effect on cytokinesis in wild type or RhoK mutant embryos, ruling out the calmodulin/MLCK pathway as the missing regulator of cytokinesis in the C. elegans early embryo.
Keywords: calmodulin, C. elegans, cytokinesis, MLCK, phosphorylation
1.Introduction
Phosphorylation on the regulatory light chain of myosin II (mRLC) stimulates myosin II force production thereby regulating many actomyosin based contractile events including the constriction of the contractile ring in cytokinesis [1]. Three kinases have been implicated in mRLC phosphorylation during cytokinesis; the calmodulin-dependent Myosin Light Chain Kinase (MLCK), and two RhoGTPase effector kinases: Rho-dependent kinase (RhoK) and citron kinase (CitK) [1,2]. MLCK is hypothesized to contribute to mRLC phosphorylation during cytokinesis because Ca2+/calmodulin-activated MLCK is a known regulator of a similar actomyosin based contractile system, that of smooth muscle [3]. Moreover, direct evidence suggests that calmodulin and MLCK may be involved in activating the contractile ring. Both calmodulin and some isoforms of MLCK are localized to the cleavage furrow in HeLa cells [4,5] [6,7]. Loss of calmodulin or application of calmodulin inhibitors disrupts or prevents cytokinesis [7,8] and a MLCK inhibitor causes cytokinesis defects in LLCPK1 cells and crane-fly spermatocytes [9,10].
Although CitK is essential for the completion of cytokinesis in proliferating tissues in Drosophila and in certain neuronal precursor cells in mouse [2], the most likely C. elegans CitK homologues play no role in cytokinesis [11,12]. C. elegans RhoK mutant embryos, on the other hand, have decreased mRLC phosphorylation and slowed division furrows that often fail. However, some mRLC phosphorylation remains and many cytokinesis events complete normally. Furthermore, double mutant embryos of RhoK and the opposing phosphatase have wild type furrowing and levels of mRLC phosphorylation [11], suggesting that other kinases, perhaps MLCK, contribute to mRLC phosphorylation during cytokinesis.
There is as yet no non-muscle MLCK described in C. elegans, so we used RNA mediated interference (RNAi) to determine the role of the upstream regulator of MLCK, the calcium sensor calmodulin, as well as candidate MLCKs, during cytokinesis in early C. elegans embryos. We found that RNAi against calmodulin did not cause cytokinesis defects in early embryos, although subtle defects in chromosome segregation were observed. Nor were enhanced cytokinesis defects observed when calmodulin and MLCK candidates were depleted simultaneously or in the background of a RhoK mutant. These results suggest that neither calmodulin nor its effector kinases, including MLCK, regulate cytokinesis in C. elegans early embryos.
2. Methods and Materials
C. elegans strains and alleles
The following strains were used: Bristol N2 strain (wild type), WH0280 unc-119(ed3); ojEx38 [cmd-1::gfp, unc-119(ed3)+] (cmd-1::gfp), HR863 let-502(sb106) (RhoK mutant), BC3541 dpy-18(e364)/eT1 III; sDf52 unc-46(e177)/eT1[let-500(s2165)] (cmd-1 deficiency strain), NL2099 rrf-3(pk1426) (RNAi sensitive strain), TY3558 (unc-119(ed3) ruIs32[pie-1::GFP::his-11] III; ojIs1[tbb-2::GFP])(histone gfp and tubulin gfp), SU180 itr-1(jc5) (ITR-1 mutant), SU93 jcls1 [ajm-1::gfp] (cell junctional marker strain), and SU188 itr-1(jc5); jcIs1 [ajm-1::gfp]) (ITR-1 mutant crossed with the cell junctional marker strain). Standard procedures were used for the culturing and handling of strains [13]. The temperature sensitive let-502(sb106) mutant strain was cultured at 16°C and shifted to 25°C 1-2 hours before imaging and the cold sensitive mutant strain itr-1(jc5) was cultured at 20°C and shifted to 16°C for 24 hours before imaging.
RNA mediated interference
DNA templates for in vitro transcription of RNA (Ambion) were generated by PCR, using primers specific to the gene of interest also containing an RNA polymerase initiation site, on a cDNA clone, yk494f9 covering T21H3.3 (cmd-1) and genomic DNA covering the predicted open reading frame of C13C12.1 (cal-1) (we determined a different start site for cal-1 than indicated on Wormbase (Supplemental Data), so our PCR fragment only partially covers the predicted ORF), C18E9.1 (cal-2), M02B7.6 (cal-3) and T07G12.1 (cal-4). DNA Templates for MLCK and CitK candidate genes were amplified from plasmids containing 1 – 1.5 Kb of target sequence [14]. Target sequence for MLCK candidates was from W06H8.8, ZK617.1, K07A9.2, K11E8.1, K12C11.4, ZC373.4, and C09D1.1. Citron domain targets included F59A6.5, K08B12.5, T08G5.5, W02B8.2, ZC404.9, and ZC504.4a. dsRNA was injected into young adult hermaphrodites at 0.5-5 ng/μl [15].
Western blot
Embryos were dissected from 60 adults, boiled in Laemmli Buffer (BioRad) plus 5% 2-Mercaptoethanol (BioRad) 5', and vortexed 10' with an equal volume of 425-600 μM glass beads (Sigma). Samples were resolved by SDS-PAGE and transferred and fixed to membrane [16], then immunoblotted with antibodies at these concentrations: mouse anti-actin c4 (Sigma) 1:1333 and mouse anti-calmodulin (Zymed) at 1:333. Secondary goat anti-mouse HRP (BioRad) antibodies were detected with chemilumenescent reagents (ECL). Membranes were exposed to Biomax film (Kodak). Intensity measurements were made in Adobe Photoshop.
Live imaging
Embryos were mounted on slides and imaged with time-lapse differential interference contrast (DIC) [17]. CMD::GFP and histone::GFP; tubulin::GFP embryos were imaged by multiphoton excitation [18] and were minimally processed in Image J.
Creation of CMD-1::GFP
A full length cmd-1 genomic DNA PCR fragment was cloned into the pFJ1.1 plasmid (pFJ1.1 was constructed by adding an unc-119 rescuing fragment to the pie-1 expression vector [19]) at the spe-1 restriction site using conventional methods and introduced into unc-119(ed3) worms by biolistic bombardment [20].
Embryo Inhibitor Studies
Embryos were exposed to calmodulin inhibitors at stages between meiosis I and II when they are still permeable to dyes and other molecules, presumably because the eggshell is not yet fully formed (unpublished observations)[21]. Embryos were dissected from adults in 3 μl inhibitor solution (calmidazolium chloride (calm) (Sigma), Compound 48/80 (c48/80) (MP Biomedicals), and N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide (W-7) (MP Biomedicals)) on a glass slide, covered by a cover slip suspended on a ring of petroleum jelly, and subjected to time-lapse DIC microscopy.
3. Results and Discussion
Depletion of calmodulin in C. elegans embryos
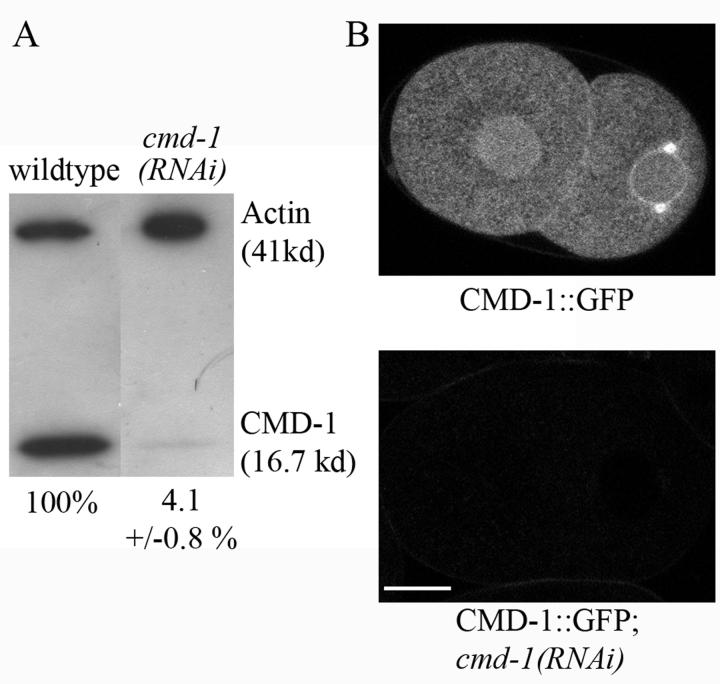
There is no clear MLCK homologue in C. elegans yet described. To determine if the calmodulin/MLCK pathway acts in cytokinesis in C. elegans, we depleted the single C. elegans calmodulin protein in embryos. The efficiency of depletion was assessed by Western blotting using antibodies raised against Dictyostelium calmodulin, which recognizes bovine brain calmodulin (not shown) and C. elegans calmodulin in this assay (Fig. 1A). Quantification of bands, using actin as a loading control, indicated that CMD-1 was reduced by 95.9 ± 0.8% in RNAi treated embryos. GFP fluorescence was also eliminated by cmd-1(RNAi) in embryos expressing a cmd-1::gfp construct (Fig. 1B).
Fig. 1.
CMD-1 is significantly reduced in cmd-1 RNAi treated embryos. A) Western Blot. Numbers indicate the percentage of calmodulin in the sample, normalized to actin. B) Fluorescence images of two-cell embryos expressing CMD-1::GFP. Fluorescence is no longer observed in the cmd-1(RNAi) embryo. Bar 10 μM.
Despite this significant reduction of CMD-1, developmental events appeared mostly normal in cmd-1(RNAi) early embryos (Table 1), though, as previously reported, 100% arrested at mid embryogenesis [22],(Supplemental Fig. S1). Simultaneous depletion of four calmodulin-like proteins (CAL) [22,23] with CMD-1 also does not result in cytokinesis defects (Table 1).
Table 1.
RNAi phenotypes of calmodulin and MLCK candidates.
| Genotype | % Cytokinesis defects in the early embryo |
|---|---|
| N2 | 2 (90)1 |
| cmd-1(RNAi) | 4 (66) |
|
cmd-1(RNAi); cal-1(RNAi); cal-2(RNAi); cal-3(RNAi); cal-4(RNAi)2 |
3 (32) |
| let-502(sb106)3 | 20 (44) |
| let-502(sb106);cmd-1(RNAi) | 305 (25) |
|
let-502(sb106);ttn-1(RNAi); unc-22(RNAi);cmk-1(RNAi); unc-43(RNAi);dapk-1(RNAi); unc-89(RNAi);ZC373.4(RNAi)4 |
185 (>20) |
| itr-1(jc5)6 | − (12) |
| itr-1(jc5);cmd-1(RNAi) | − (17) |
(N), number of embryos scored.
cal-1-4, calmodulin-like genes.
let502(sb106), RhoK loss of function mutant.
ttn-1, unc-22, cmk-1, unc-43, dapk-1, unc-89, and ZC373.4, MLCK candidates. Several MLCK candidates have been described as homologues of genes other than non muscle MLCK (see Table 2), but all were depleted to ensure that no role was missed due to functional redundancy.
not significantly different from let-502(sb106).
itr-1(jc5), IP3R loss of function mutant.
To disrupt calmodulin further, CMD-1 was depleted by RNAi in a deficiency strain in which the chromosomal deletion sDF52 removes one copy of cmd-1 and nearby genes, and in the RNAi sensitive mutant rrf-3(pk1426)[24]. In addition, embryos were treated with pharmacological calmodulin inhibitors (see Methods and Materials). These treatments all tended to increase polar body extrusion (meiosis) failures but did not cause significant specific defects in mitotic cytokinesis (Supplemental Fig. S2).
Calmodulin, a calcium sensor, acts on many targets only when bound to calcium. IP3 receptor-controlled release of calcium from the endoplasmic reticulum (ER) is the source of many cytoplasmic calcium transients [25]. A mutant of the single C. elegans IP3 receptor, itr-1(jc5), has defects in epithelial cell migrations during embryo enclosure but no noticeable early embryonic defects at the non-permissive temperature [26]. itr-1(jc5) embryos treated with cmd-1 RNAi showed more severe and frequent cell migration defects (as assessed in embryos expressing AJM-1::GFP, a cell junction marker[27]) (Supplemental Fig. S3), but did not have cytokinesis defects (Table 1). We conclude that disruption of ITR-1-dependent calcium release does not sensitize embryos to a loss of calmodulin in the early embryo.
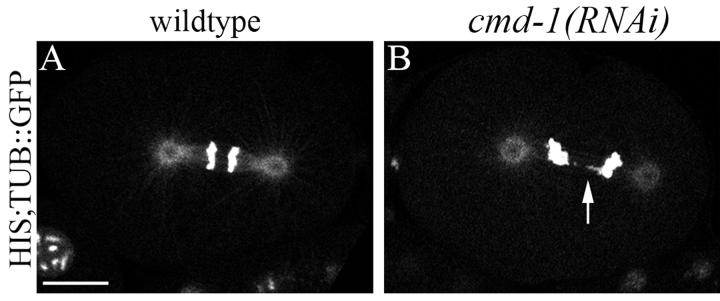
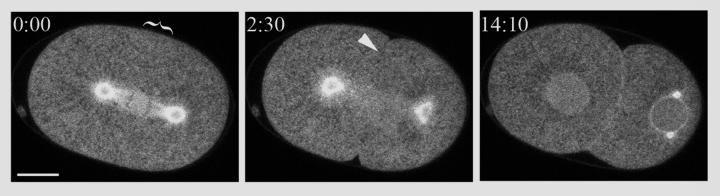
While calmodulin has no role in cytokinesis, we found that depletion of CMD-1 does cause subtle chromosome segregation defects in early embryos, confirming previous reports [12]. To look more closely at DNA segregation, we depleted CMD-1 in worms expressing GFP-tagged histone and tubulin. 45% of early embryo cell divisions had lagging and disorganized chromosomes, although they most often segregated successfully (Fig. 2 and Supplemental Video 1). This defect is reminiscent of that seen in yeast, where disruption of calmodulin at the spindle pole body leads to broken spindles, chromosome segregation defects, and mitotic arrest [28-31]. While C. elegans early embryos lacking nearly all calmodulin have grossly normal looking spindles (by light microscopy, not shown) and do not arrest, there are likely underlying spindle defects and, whereas disruption of spindle function and chromosome segregation trigger cell cycle arrest checkpoints in yeast, activation of the C. elegans early embryo spindle checkpoint causes only a moderate delay [32]. Further supporting these conclusions, GFP tagged CMD-1 does not accumulate at the furrow, but does accumulate on the spindle and centrosomes (as well as to the interphase nuclear membrane and the borders of abutting cells) (Fig. 3 and Supplemental Video 2).
Fig. 2.
Chromosome segregation is defective in cmd-1(RNAi) embryos. Fluorescence images of anaphase embryos expressing HIS::GFP;TUB::GFP. A) Untreated embryo. B) cmd-1(RNAi) embryo containing lagging chromosomes (arrow). Bar 10 μM.
Fig. 3.
CMD-1 localizes to discrete structures C. elegans embryos. Fluorescent images of a cmd-1::gfp expressing embryo. 0m:00s, anaphase. 2:30, initiation of cleavage furrow ingression. 14:10, 2 cell embryo. Anterior cell (left), anaphase, posterior cell (right), interphase. CMD-1 is present at the centrosome and spindle but no accumulation is seen at the presumptive or ingressing furrow (parentheses, arrowhead). Bar 10 μM.
Calmodulin and candidate MLCKs do not function redundantly with RhoK
The C. elegans RhoK, LET-502, is the only kinase shown thus far to regulate myosin II activation in the furrow in C. elegans [11] but other kinases likely act redundantly in the regulation of this tightly controlled process. The calmodulin/MLCK pathway is not necessary for contractile ring activation in C. elegans, but might function redundantly with the RhoK pathway. However, when calmodulin was depleted in a RhoK mutant background, there was no enhancement of cytokinesis failures (Table 1). MLCK candidate homologues, determined by comparison of C. elegans peptides with the protein sequence and domain organization of known MLCKs (Table 2), do not have reported early embryonic defects when depleted in wild type [12], and none enhance early embryonic phenotypes when depleted in pools of 3-7 in a RhoK mutant background (Table 1). Given these results, it is unlikely that a calmodulin-activated MLCK is acting in concert with RhoK to activate myosin in the contractile ring of C. elegans embryos.
Table 2.
Determination of C. elegans MLCK candidate proteins.
| Myosin Light Chain Kinases | Isoform1 Size(s) (kDa) |
IG/FN1 Domains |
Average % AA Identity to Vertebrate MLCKs (kinase domain)1, 2 |
Regulatory Domain1 |
|---|---|---|---|---|
| Vertebrate smooth muscle/ non-muscle MLCK |
108, 210 | 0-9/1 | calmodulin | |
|
Drosophila Stretchin-MLCK short isoforms |
85, 165 | 0-7/1 | calmodulin | |
| Dictyostelium MLCK | 33 | 0/0 | phosphorylation | |
| C. elegans MLCK candidates3 | ||||
| ttn-1(short isoforms) | 77, 80 | 4/0 | 50.5 | ? |
| unc-22 | 750 | 27/31 | 49.5 | S100A124 |
| cmk-1 | 39 | 0/0 | 39.25 | calmodulin5 |
| unc-43 | 16-80 | 0/0 | 35.25 | calmodulin5 |
| dapk-1 | 161 | 0/0 | 42.25 | ? |
| ZC373.4 | 135 | 0/0 | 50.25 | calmodulin5 |
| unc-89(short isoforms) | 156, 157 | 1/1 | 35.5 | calmodulin5 |
Non-muscle MLCKs usually contain one or more immunoglobulin (IG) and fibronectin (FN) type III domains, are often isoforms of larger proteins, and usually contain a calmodulin binding regulatory domain in addition to a kinase domain [1,30].
Average percent identity to mouse, human, and rabbit smooth muscle/non-muscle MLCK.
Domain organizations of C. elegans candidates were determined through the NCBI conserved domain database (CCD).
The S100A12 binding domain is found in a member of the S100 family of calcium binding proteins [34].
The calmodulin binding domain sequence is short and not highly conserved so the stringency of blast searches was relaxed (expected E value threshold of 1.0) to generate these matches.
Previous results suggested that citron kinases have no role in C. elegans embryo cytokinesis [11,12]. We confirmed these results and also depleted all citron domain containing proteins in the RhoK background but saw no effect on cytokinesis when CitK candidates were depleted simultaneously in wild type or the RhoK mutant, although RNAi against the CitK domain containing gene F59A6.5 resulted in altered post-cleavage cortical activity (not shown).
We have shown that, unexpectedly, the Ca2+/calmodulin MLCK pathway is not involved in regulation of the contractile ring in cytokinesis in C. elegans embryos, although calmodulin may have a modulating role in spindle organization. The missing kinase that likely acts with RhoK to phosphorylate mRLC in the contractile ring in C. elegans embryos must therefore be different from canonical CitKs or MLCKs that control cytokinesis in other cell types.
Supplementary Material
Table 3.
C. elegans Citron Kinase Candidates.
|
C. elegans Citron Kinase Candidates |
Citron Homology Domain (CNH)1 |
Kinase Domain2 |
|---|---|---|
| F59A6.5 | Yes | No |
| tag-59 | Yes | Yes |
| T08G5.5 | Yes | No |
| W02B8.2 | Yes | No |
| gck-2 | Yes | Yes |
| mig-15 | Yes | Yes |
Citron Kinase candidates were determined by the presence of the CNH domain (defined by Pfam).
As annotated on WormBase and the NCBI conserved domain database (CDD). Note that not all CNH domain containing candidates also have kinase domains.
Acknowledgements
We wish to thank P. Mains for the let-502(sb106) strain, P. McDonel and B. Meyer for the histone, tubulin GFP strain, the C. elegans Genetics Center for the cmd-1 deficiency and RNAi sensitive strains, The Genetic Toolkit (both funded by the NIH National Center for Research Resources), and Yugi Kohara for the yk494f9 cDNA clone, and J. Plastino for advice in editing. This project was funded by NIH grants GM 057583 to JGW and GM 058038 to JDH.
Footnotes
Abbreviations: mRLC, myosin Regulatory Light Chain; MLCK, Myosin Light Chain Kinase; RhoK, Rho-dependent Kinase; CitK, Citron Kinase; RNAi, RNA mediated interference; DIC, Differential Interference Contrast; IP3, Inositol 1,4,5 trisphosphate.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kamm KE, Stull JT. Dedicated myosin light chain kinases with diverse cellular functions. J Biol Chem. 2001;276:4527–30. doi: 10.1074/jbc.R000028200. Epub 2000 Nov 28. [DOI] [PubMed] [Google Scholar]
- 2.Matsumura F. Regulation of myosin II during cytokinesis in higher eukaryotes. Trends Cell Biol. 2005;15:371–7. doi: 10.1016/j.tcb.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Satterwhite LL, Pollard TD. Cytokinesis. Curr Opin Cell Biol. 1992;4:43–52. doi: 10.1016/0955-0674(92)90057-j. [DOI] [PubMed] [Google Scholar]
- 4.Chew TL, Wolf WA, Gallagher PJ, Matsumura F, Chisholm RL. A fluorescent resonant energy transfer-based biosensor reveals transient and regional myosin light chain kinase activation in lamella and cleavage furrows. J Cell Biol. 2002;156:543–53. doi: 10.1083/jcb.200110161. Epub 2002 Jan 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poperechnaya A, Varlamova O, Lin PJ, Stull JT, Bresnick AR. Localization and activity of myosin light chain kinase isoforms during the cell cycle. J Cell Biol. 2000;151:697–708. doi: 10.1083/jcb.151.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erent M, Pagakis S, Browne JP, Bayley P. Association of calmodulin with cytoskeletal structures at different stages of HeLa cell division, visualized by a calmodulin-EGFP fusion protein. Mol Cell Biol Res Commun. 1999;1:209–15. doi: 10.1006/mcbr.1999.0137. [DOI] [PubMed] [Google Scholar]
- 7.Yu YY, et al. The association of calmodulin with central spindle regulates the initiation of cytokinesis in HeLa cells. Int J Biochem Cell Biol. 2004;36:1562–72. doi: 10.1016/j.biocel.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Li CJ, Heim R, Lu P, Pu Y, Tsien RY, Chang DC. Dynamic redistribution of calmodulin in HeLa cells during cell division as revealed by a GFP-calmodulin fusion protein technique. J Cell Sci. 1999;112:1567–77. doi: 10.1242/jcs.112.10.1567. [DOI] [PubMed] [Google Scholar]
- 9.Murthy K, Wadsworth P. Myosin-II-Dependent Localization and Dynamics of F-Actin during Cytokinesis. Curr Biol. 2005;15:724–31. doi: 10.1016/j.cub.2005.02.055. [DOI] [PubMed] [Google Scholar]
- 10.Silverman-Gavrila RV, Forer A. Effects of anti-myosin drugs on anaphase chromosome movement and cytokinesis in crane-fly primary spermatocytes. Cell Motil Cytoskeleton. 2001;50:180–97. doi: 10.1002/cm.10006. [DOI] [PubMed] [Google Scholar]
- 11.Piekny AJ, Mains PE. Rho-binding kinase (LET-502) and myosin phosphatase (MEL-11) regulate cytokinesis in the early Caenorhabditis elegans embryo. J Cell Sci. 2002;115:2271–82. doi: 10.1242/jcs.115.11.2271. [DOI] [PubMed] [Google Scholar]
- 12.Sonnichsen B, et al. Full-genome RNAi profiling of early embryogenesis in Caenorhabditis elegans. Nature. 2005;434:462–9. doi: 10.1038/nature03353. [DOI] [PubMed] [Google Scholar]
- 13.Brenner S. The Genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamath RS, et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–7. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- 15.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–11. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 16.Hulen D, Baron A, Salisbury J, Clarke M. Production and specificity of monoclonal antibodies against calmodulin from Dictyostelium discoideum. Cell Motil. Cytoskeleton. 1991;18:113–22. doi: 10.1002/cm.970180206. [DOI] [PubMed] [Google Scholar]
- 17.Skop AR, White JG. The dynactin complex is required for cleavage plane specification in early Caenorhabditis elegans embryos. Curr Biol. 1998;8:1110–6. doi: 10.1016/s0960-9822(98)70465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wokosin DL, Squirrell JM, Eliceiri KW, White JG. Optical workstation with concurrent, independent multiphoton imaging and experimental laser microbeam capabilities. Rev. Sci. Instrum. 2003;74:1–9. doi: 10.1063/1.1524716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strome S, Powers J, Dunn M, Reese K, Malone CJ, White J, Seydoux G, Saxton W. Spindle dynamics and the role of gamma-tubulin in early Caenorhabditis elegans embryos. Mol Biol Cell. 2001;12:1751–64. doi: 10.1091/mbc.12.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Praitis V, Casey E, Collar D, Austin J. Creation of low-copy integrated transgenic lines in Caenorhabditis elegans. Genetics. 2001;157:1217–26. doi: 10.1093/genetics/157.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wood W. Embryology. In: Wood W, editor. The Nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory Press; 1988. pp. 215–241. [Google Scholar]
- 22.Karabinos A, Bussing I, Schulze E, Wang J, Weber K, Schnabel R. Functional analysis of the single calmodulin gene in the nematode Caenorhabditis elegans by RNA interference and 4-D microscopy. Eur J Cell Biol. 2003;82:557–63. doi: 10.1078/0171-9335-00347. [DOI] [PubMed] [Google Scholar]
- 23.Salvato M, Sulston J, Albertson D, Brenner S. A novel calmodulin-like gene from the nematode Caenorhabditis elegans. J Mol Biol. 1986;190:281–9. doi: 10.1016/0022-2836(86)90002-1. [DOI] [PubMed] [Google Scholar]
- 24.Simmer F, Tijsterman M, Parrish S, Koushika SP, Nonet ML, Fire A, Ahringer J, Plasterk RH. Loss of the putative RNA-directed RNA polymerase RRF-3 makes C. elegans hypersensitive to RNAi. Curr Biol. 2002;12:1317–9. doi: 10.1016/s0960-9822(02)01041-2. [DOI] [PubMed] [Google Scholar]
- 25.Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32:235–49. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- 26.Thomas-Virnig CL, Sims PA, Simske JS, Hardin J. The inositol 1,4,5-trisphosphate receptor regulates epidermal cell migration in Caenorhabditis elegans. Curr Biol. 2004;14:1882–7. doi: 10.1016/j.cub.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Koppen M, Simske JS, Sims PA, Firestein BL, Hall DH, Radice AD, Rongo C, Hardin JD. Cooperative regulation of AJM-1 controls junctional integrity in Caenorhabditis elegans epithelia. Nat Cell Biol. 2001;3:983–91. doi: 10.1038/ncb1101-983. [DOI] [PubMed] [Google Scholar]
- 28.Moser MJ, Flory MR, Davis TN. Calmodulin localizes to the spindle pole body of Schizosaccharomyces pombe and performs an essential function in chromosome segregation. J Cell Sci. 1997;110:1805–12. doi: 10.1242/jcs.110.15.1805. [DOI] [PubMed] [Google Scholar]
- 29.Sundberg HA, Goetsch L, Byers B, Davis TN. Role of calmodulin and Spc110p interaction in the proper assembly of spindle pole body compenents. J Cell Biol. 1996;133:111–24. doi: 10.1083/jcb.133.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun GH, Hirata A, Ohya Y, Anraku Y. Mutations in yeast calmodulin cause defects in spindle pole body functions and nuclear integrity. J Cell Biol. 1992;119:1625–39. doi: 10.1083/jcb.119.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stirling DA, Rayner TF, Prescott AR, Stark MJ. Mutations which block the binding of calmodulin to Spc110p cause multiple mitotic defects. J Cell Sci. 1996;109:1297–310. doi: 10.1242/jcs.109.6.1297. [DOI] [PubMed] [Google Scholar]
- 32.Encalada SE, Willis J, Lyczak R, Bowerman B. A spindle checkpoint functions during mitosis in the early Caenorhabditis elegans embryo. Mol Biol Cell. 2005;16:1056–70. doi: 10.1091/mbc.E04-08-0712. Epub 2004 Dec 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Champagne MB, Edwards KA, Erickson HP, Kiehart DP. Drosophila stretchin-MLCK is a novel member of the Titin/Myosin light chain kinase family. J Mol Biol. 2000;300:759–77. doi: 10.1006/jmbi.2000.3802. [DOI] [PubMed] [Google Scholar]
- 34.Donato R. Intracellular and extracellular roles of S100 proteins. Microsc Res Tech. 2003;60:540–51. doi: 10.1002/jemt.10296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.