Abstract
In most insects, sperm transferred by the male to the female during mating are stored within the female reproductive tract for subsequent use in fertilization. In Drosophila melanogaster, male accessory gland proteins (Acps) within the seminal fluid are required for efficient transfer and subsequent accumulation of sperm in the female's sperm storage organs. To determine the events within the female reproductive tract that occur during sperm storage, and the role that Acps and sperm play in these events, we identified morphological changes that take place during sperm storage in females mated to wild-type, Acp-deficient or sperm-deficient males. A reproducible set of morphological changes occurs in a wild-type mating. These were categorized into 10 stereotypic stages. Sperm are not needed for progression through these stages in females, but receipt of Acps is essential for progression beyond the first few stages of morphological changes. Furthermore, females that received small quantities of Acps reached slightly later stages than females that received no Acps. Our results suggest that timely morphological changes in the female reproductive tract, possibly muscular in nature, may be needed for successful sperm storage, and that Acps from the male are needed in order for these changes to occur.
Keywords: Sperm, uterus, mating plug, muscle contraction, fertility, seminal proteins
1. Introduction
Sperm storage by inseminated females is a crucial step of sexual reproduction (e.g. reviewed in: Neubaum and Wolfner, 1999a; Bloch Qazi et al., 2003). Sperm are stored in specialized regions or organs within the female reproductive tract until they are needed for fertilization. The sequestration of sperm in storage allows females to retain sperm after a mating, rather than having the sperm mass swept out of the female reproductive tract by the passage of the first egg (in Drosophila: Kaufman and Demerec, 1942; Lefevre and Jonsson, 1962; Fowler, 1973; Gilbert, 1981; Bertram et al., 1996). Storage of sperm also extends the amount of time when sperm are available for fertilization, allows for the temporal decoupling of insemination and fertilization, permits competition between the ejaculates of males (Parker, 1970) and/or sperm preference/choice by the female (Thornhill, 1983; Birkhead and Møller, 1993; Eberhard, 1996; Birkhead, 1998) in cases of multiple-mating. Sperm storage can also extend the duration of female egg-laying and refractoriness to mating (in Drosophila: Manning, 1962, 1967; Kalb et al., 1993, Liu and Kubli, 2003, Peng et al., 2005). Yet, despite the importance of sperm storage to reproduction, the proximate mechanisms by which it occurs - and the relative roles of male and female contributions to its success - are not well understood. We have used microscopy of wild-type and transgenic Drosophila melanogaster to investigate these mechanisms, and their triggers, in this insect.
D. melanogaster males transfer up to ∼4000 sperm to females during a typical 20-minute mating (Lefevre and Jonsson, 1962; Fowler, 1973; Gilbert 1981). Approximately 1000 of these sperm are stored (Kaufman and Demerec, 1942, Fowler, 1973; Gilbert, 1981; Tram and Wolfner, 1999; Bloch Qazi and Wolfner, 2003; Iida and Cavener, 2004), although the exact number varies between strains (L. McGraw, M. Bloch Qazi, Ravi Ram K., and M.F.W., unpublished observations). Sperm are stored in the female's seminal receptacle (SR) and her paired spermathecae (St) (Fig. 1) for up to two weeks. Sperm storage begins before mating is complete and peak storage is reached by ∼1 hr post-mating (Bloch Qazi and Wolfner, 2003). By ∼5min after the start of mating (ASM), ejaculatory bulb/duct secretions that will form the waxy posterior mating plug are detected in females (Bairati, 1968; Ludwig et al., 1991; Lung and Wolfner, 2001). These secretions coagulate in the posterior uterus (Bairati and Perotti, 1970; Lung and Wolfner, 2001), though the mating plug does not completely occlude the mated female's reproductive tract (Bairati 1968). It is thought that this plug may assist in sperm movement within the uterus and/or may also prevent the loss of sperm and seminal secretions from the mated female (Bairati 1968). At ∼7–10 min ASM, sperm, male accessory gland proteins (Acps), and other seminal fluid proteins are detected within the female reproductive tract (Bairati, 1968; Meikle et al., 1990; Monsma et al., 1990; Lung and Wolfner, 2001; Ravi-Ram-etal-2005). By the end of mating (∼20min ASM), the uterus and vagina are rounded and turgid (e.g. Nonidez, 1920; Patterson and Stone, 1952), and a discrete mass of sperm is visible in the anterior uterus near the openings to the SR and St (reviewed in Bloch Qazi et al., 2003; Fowler, 1973; Gromko, 1984). Just posterior to this sperm mass is the mating plug, whose anterior portion contains Acps (Lung and Wolfner, 2001) and whose posterior part was described above.
Figure 1.
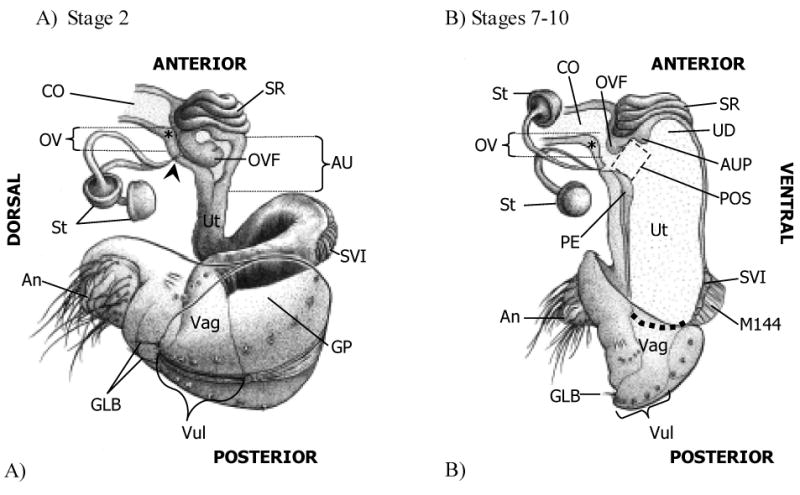
Conformation of the lower reproductive tract of wild type (Oregon R) Drosophila melanogaster females at early (A) and late (B) times in sperm storage. (A) At the start of ejaculate transfer (Stage 2), the uterus (Ut) is contracted and forms a loop at the specialized vaginal intima (Miller 1950; SVI). In addition, the anterior uterus (AU) is bent laterally above the SVI such that, in the drawing, the SVI and vagina (Vag) appear to project toward the reader at a 45 degree angle to the plane of the page/AU. Within the AU, the oviduct valve flap (OVF) is curled posterio-ventrally, covering the openings to the spermathecal ducts (filled arrowhead; note that only the AU and common oviduct (CO) are shown in cross-section in this drawing, the rest of the reproductive tract is shown only from the exterior.). (B) During the later stages of sperm storage (Stages 7–10), the uterus is fully expanded and turgid. In the anterior uterus, the pre-oviduct space (POS) forms between the (anterior) papillate elevation (Miller 1950; PE), the anterior uterus projection (AUP), and the oviduct valve flap, which has uncurled. In this drawing, for clarity, the oviduct valve (OV) is shown open, although in most instances the OVF contacts a ridge in the dorsal oviduct wall (*) just above the openings to the spermathecal ducts, closing the OV. The dashed line denotes the margin between the Ut and Vag. An, anus; GLB, gonopod long bristle(s); GP, gonopod plate; M144, muscle 144 (Miller 1950) attached to the SVI; St, spermatheca; SR, seminal receptacle; UD, uterine dome; Vul, vulva (The female accessory glands, or parovaria, have been omitted from these drawings. Drawings by Anthony Yori).
How sperm reach the openings of the SR and St at the anterior uterus is not known, although a combination of both male and female factors likely aid sperm movement through the female reproductive tract (reviewed in Ward, 1998 and Neubaum and Wolfner, 1999a;De Vries, 1964;Gromko, 1984; Arthur et al., 1998). Products of the male's accessory glands were suggested to be important for the storage and/or utilization of sperm in females (Lefevre and Jonsson, 1962; Hihara, 1981; Fuyama, 1983) and subsequent genetic/transgenesis studies showed that Acps are critical for these processes (Kalb et al., 1993; Tram and Wolfner, 1999; Xue and Noll, 2000; Bloch Qazi and Wolfner, 2003). Females mated to males that provide less than 1% of wild-type amounts of Acps stored less than 10% as many sperm as normal (Tram and Wolfner, 1999), and at least one Acp has been implicated in this process (Neubaum and Wolfner,1999b;Bloch Qazi and Wolfner, 2003). The few sperm stored are not utilized for fertilization in the absence of Acps (Xue and Noll, 2000). Although accessory gland proteins are thus clearly important, other types of macromolecules have been reported among accessory gland secretions of some insects (reviewed in Gillott 2003; this has not been examined in detail in Drosophila). In some Drosophila strains tested for Acp function, accessory glands are absent or are incapable of making protein (including, presumably, enzymes needed to synthesize non-proteinaceous macromolecules). Thus it is possible that non-proteinaceous products of accessory glands might also have reproductive effects; however, the present study could not differentiate roles for such molecules. Although the means by which accessory gland products could affect sperm storage or utilization is not known, Acp-based modulation of vesicle-release in the female reproductive tract (Heifetz and Wolfner, 2004) suggests that Acps might modulate muscle contraction within the female reproductive tract to assist in moving the sperm mass towards storage sites. Circular muscles that surround the uterus, as well as longitudinal muscles that run the length of the uterus and vagina (often connecting the uterus to the exoskeleton; Miller, 1950) could assist in this. However, the stages in the movement of sperm into storage were unknown, as was their dependence (if any) on the presence of sperm or other male-provided components of the ejaculate.
Here we present the first detailed description of sperm movement through the D. melanogaster female reproductive tract into storage. We identified a series of 10 reproducible and sequential morphological stages that occur in the uterus and vagina during and after a wild-type mating, and determined the location of sperm at each stage. To dissect the contributions of sperm, Acps and non-Acp/non-sperm stimuli from mating in inducing these changes in females, we then examined the progression through the ten stages in females mated to males that transferred sperm but little or no Acps (Kalb et al., 1993; Xue and Noll, 2000), or no sperm but wild-type amounts of Acps (Boswell and Mahowald, 1985; Kalb et al., 1993), or neither sperm nor Acps (Kalb et al., 1993). We found that Acps are required for the morphological changes we observed during sperm storage. In contrast, sperm are not needed for these changes, nor are they (or non-Acp/non-sperm stimuli) sufficient to trigger the morphological changes in the uterus and vagina that accompany and may mediate sperm entry into storage in D. melanogaster.
2. Materials and methods
2.1. D. melanogaster strains and rearing
All flies were maintained and crossed on standard glucose-yeast media at 23°C ± 2° and a 12L:12D hr cycle. Females were collected as virgins within 6 hours of eclosion and males were collected within 24 hours of eclosion, on ice. Flies were aged individually for 4–6 days prior to experimental crosses. We used the Oregon R strain as our wild-type. Spermless (son-of-tudor; SOT) males were the sons of Oregon R males and virgin tudor females (tud1 bw sp/tud1 bw sp;Boswell and Mahowald, 1985). DTA-E males, which produce neither Acps nor sperm, and DTA-D males, which produce sperm but only 1% of wild-type amounts of Acps, were as inKalb et al., 1993. Paired-rescue (prd-rescue; prd2.45Bprd-GsbN+PrdC/SM1;ry) males, which produce sperm but no Acps were a kind gift from L. Xue and M. Noll and were generated as in Xue and Noll, 2000. Western blotting with anti-Acp antibodies (Monsma et al., 1990; Bertram et al., 1996), microscopic examination of male reproductive tract morphology and the presence of sperm, and fertility tests were used to verify that the tested males had the expected phenotype in terms of Acps and sperm. To examine the movement of sperm through the female reproductive tract, we crossed the dj-GFP transgene (Santel et al., 1997; X-linked insertion line kindly provided by Dr. B. Wakimoto) into the background of our Oregon R wild-type strain.
2.2. Experimental crosses and dissections
Using an aspirator, we transferred 5–7 male-female pairs into a vial containing medium; we recorded the time they were put together. As individual pairs began mating, we recorded the time at which they started mating and then gently removed each mating pair by aspiration to a clean empty vial. Following a randomly assigned time (between 2–70min ASM), pairs were frozen at −20°C. The interval of time between 2–70min ASM includes maximal sperm storage at ∼1 hr after the start of mating (Bloch Qazi and Wolfner, 2003), but excludes the onset of egg-laying 1.5–6 hours after the end of mating (Heifetz et al., 2000). We did not see females laying eggs during the time period of our experiments. We also recorded the time at which pairs naturally ended mating (if they had not already been frozen at timepoints during mating). Comparisons conducted both at the beginning and toward the end of our experiments showed that freezing did not affect our observations; there were no visible differences in uterus and vaginal morphology between freshly sacrificed females and those that had been frozen prior to dissection. Although we attempted to use males from all six genotypes on each day we conducted crosses, this was not always possible. To control for possible random effects of trial date, we included control Oregon R pairs on each day we conducted crosses. We did not find any effects of either trial start time or date on the results of our study.
Flies were maintained frozen for between 24 and 48 hours prior to dissection. For dissections, females were placed on their dorsum and pinned through the thorax with a minutin pin onto a dish of silicone elastomer (Sylgard 184, Dow Corning Corp., Midland, MI, USA). The male, if present, was gently removed from the female, making sure that ejaculate was not removed with the male. All dissections were conducted within a large drop of physiological saline (0.7% NaCl). Using fine-tipped forceps, the cuticle at the anterior abdomen was gently torn open. Moving toward the gonopod, the cuticle and underlying fat bodies were separated laterally. As soon as the uterus was visible within the abdominal cavity, the location of the ejaculate within the lumen of the uterus was noted to ensure that removal of the uterus and vagina from the abdomen did not disturb the ejaculate. The cuticle surrounding the gonopod was carefully detached. It was not possible to remove the gonopod from the vagina without disrupting its contents. The hindgut and common oviduct were then severed by pinching each with fine-tipped forceps. Finally, the uterus and vagina were gently lifted out of the female's abdomen with forceps, placed on a glass slide, and covered with a drop of halocarbon oil (Halocarbon Products Corp., River Edge, NJ, USA) for photographing. To avoid disturbing the contents of the uterus and vagina, no coverslip was placed over the samples. All slides were viewed using a Leica DMRA2 compound microscope (under visible light and UV illumination) and a Hamamatsu GRCA-ER digital camera. Images were captured on a Macintosh PowerPC G4 computer running Openlab 4.0.1 software (Improvision Inc., Lexington, MA, USA). Adobe Photoshop CS2 software (Adobe Systems Inc., San Jose, CA, USA) was used to prepare images for publication.
2.3. Statistical analyses
We used ordinal logistic regression to analyze our data (proportional odds logistic regression or “polr” in R software, available at www.r-project.org). We performed likelihood ratio tests of individual model parameters to determine whether stage as a function of time ASM differed among paired genotypes. For these analyses, a chi-square test was used to assess the null hypothesis that the coefficient of the interaction term ASM*Genotype was zero. Since not all genotypes had been sampled during identical time ranges (e.g. we sampled between 2–70min ASM for Oregon R and 2–50min ASM for prd-rescue), we removed data points at the extremes of the sampling times from one genotype for which there were no corresponding values at the other genotype (e.g. as in previous example, all Oregon R data points between 51min ASM and 70min ASM were removed). We also excluded stages 8–10 from Oregon R vs. SOT and Oregon R vs. DTA-E comparisons since these stages are differentiated on the basis of the location of sperm in the uterus and female sperm storage organs (males of these genotypes do not make sperm). We used a parallel lines test to check the basic assumption of ordinal logistic regression that regression coefficients (slopes) be equal at all levels of the response variable. The parallel lines test was violated in comparisons between Oregon R and flies from four mutant or transgenic lines (DTA-D, prd-rescue, DTA-E, and SOT). For those analyses we used multinomial regression, which assumes that the levels of the response variable are not ordered and estimates separate slope coefficients for each level of the response variable. Although ordinal regression is preferable to multinomial regression because information on the ordering of levels of the response variable are used to calculate regression coefficients, multinomial regression gives similar results in cases where the parallel lines test has been violated.
3. Results
3.1. Changes in reproductive tract morphology during sperm storage in Drosophila females occur in a series of defined stages
Despite intensive study of many aspects of Drosophila reproduction, there are few published descriptions of its female reproductive tract (Nonidez, 1920; Miller, 1950; Fowler, 1973; Bryant, 1978; Gromko, 1984; Alonso-Pimentel et al., 1994), and those focus on external genital morphology, and the vagina and the posterior uterus. To provide a complete framework for examining the changes that occur during sperm storage in the D. melanogaster female, we characterized in detail the structures within her lower reproductive tract. In addition to confirming previously-noted structures, we identified several new structures at the anterior end of the uterus (Fig. 1): At the base of the oviduct, the oviduct valve (OV) opens into the uterus. The valve separates the common oviduct from the uterus and consists of a flap (oviduct valve flap, OVF) and a ridge in the dorsal wall of the oviduct, just anterior to the openings to the spermathecae and parovaria (see Fig. 1; Fig. 2, Stage 7, ridge denoted by *). When the valve is closed, the OVF presses against the ridge in the dorsal oviduct wall, potentially blocking passage of substances between the uterus and oviduct. The OVF appears to be formed from a section of permanently invaginated ventral common oviduct at its point of attachment with the uterus, just dorsal to the seminal receptacle. Below the oviduct valve is the pre-oviduct space (POS), square in shape and only visible in fully expanded, late stage uteruses (Fig. 1B). The anterior uterus projection (AUP) is ventral to the pre-oviduct space and separates the pre-oviduct space from the uterine dome (UD).
Figure 2.
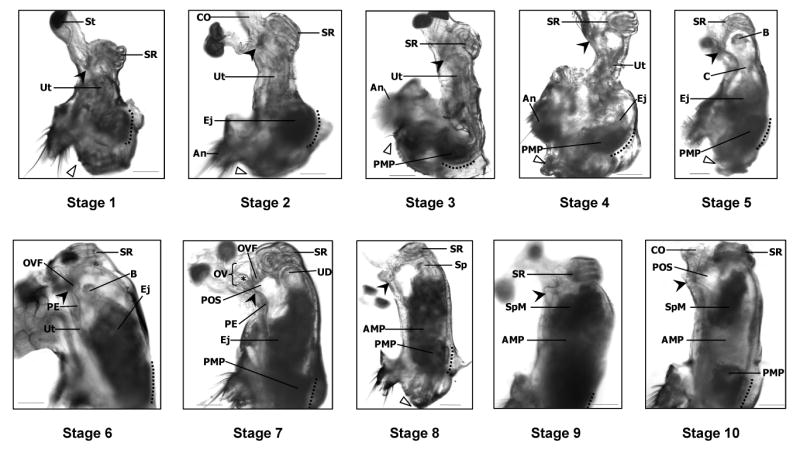
Stages of sperm transport in D. melanogaster. In virgin (Oregon R) females (Stage 1), the uterus (Ut) is highly compacted and the seminal receptacle (SR) is pulled ventrally. Soon after copulation begins (Stage 2), the uterus lengthens and ejaculate (Ej) begins to accumulate at the specialized vaginal intima (Miller 1950; SVI, to the left of dotted line). As ejaculate transfer continues (Stages 3–6), the uterus unfolds and takes on a turgid, oval shape. By Stage 7, the oviduct valve flap (OVF) has moved anteriorly, forming the top of the pre-oviduct space (POS). In Stage 8, sperm (Sp) begin to move toward the anterior uterus, leaving behind the lightly shaded anterior mating plug (AMP), visible in mid-uterus, and the posterior mating plug (PMP) adjacent to the SVI. Sperm accumulate in a mass (SpM) at the anterior uterus (Stage 9) where the openings to the sperm storage organs are located; these organs are out-of-focus in this picture. Sperm are also visible within the seminal receptacle. Finally, in Stage 10, the size of the SpM has decreased, presumably reflecting the movement of sperm into storage. The location of sperm in each stage was corroborated by examining the reproductive tracts of Oregon R females that had mated with dj-GFP (Santel et al. 1997) males, whose sperm are GFP-labeled. Filled arrowheads indicate the point where the spermathecal ducts open into the uterus. Open triangles mark the gonopod long bristle, located near the dorsal edge of the vulva. Dotted lines parallel the SVI. An, anus; B, “ball” of sperm (see text for details); C, “column” of sperm (see text for details); CO, common oviduct; St, spermatheca; OV, oviduct valve; PE, papillate elevation (Miller 1950); UD, uterus dome; *, ridge in dorsal oviduct wall which makes up part of the OV (Scale = 0.01mm).
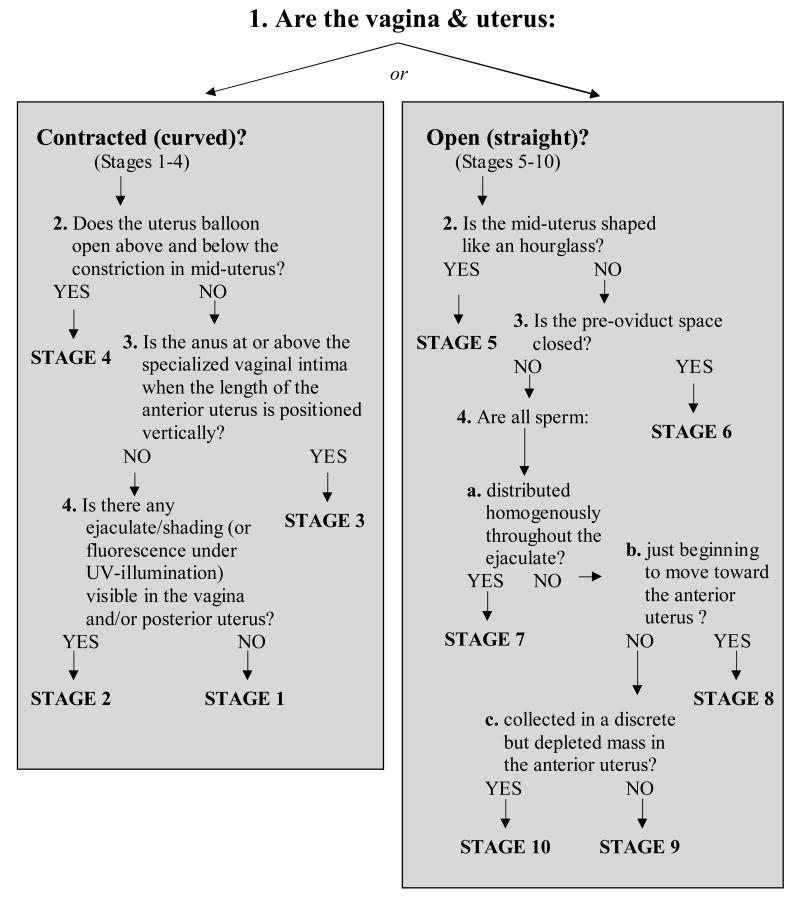
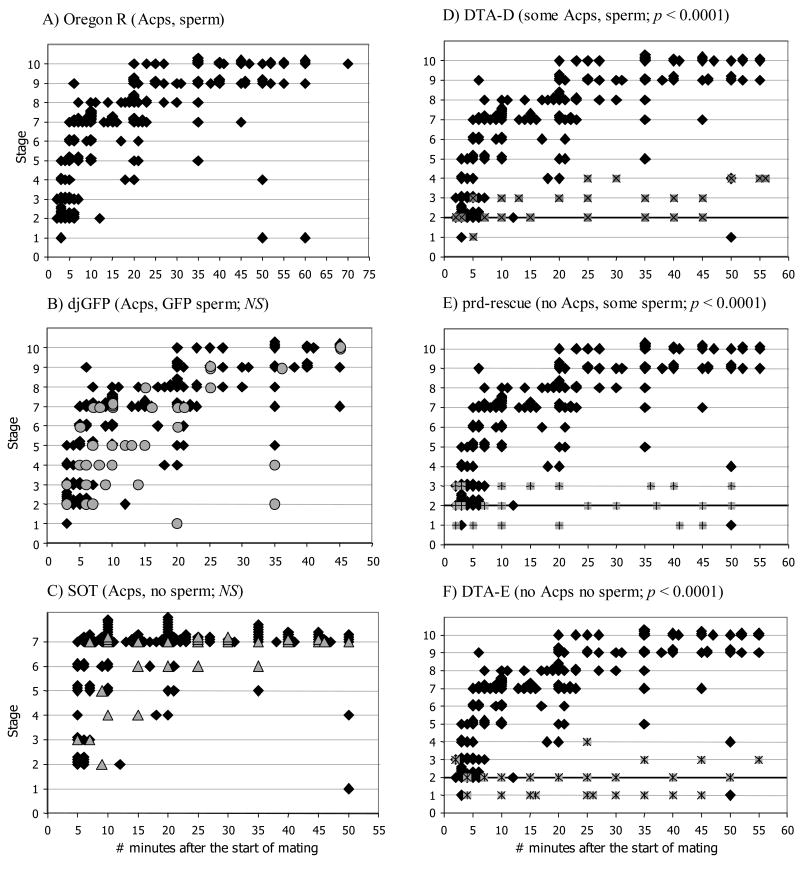
We then examined the effects of mating on the shape and position of tissues within the lower reproductive tract. We observed the reproductive tracts of Oregon R females paired with Oregon R males at different times before, during, and after mating, up to 70min ASM. We observed reproducible and stereotyped phenotypes (morphotypes) in these females. We grouped digital images of these morphotypes into 10 distinct stages based on both reproductive tract morphology and ejaculate location (Fig. 2, see descriptions below). In ordering and defining the 10 stages we assumed that: (1) the accumulation and movement of ejaculate progressed from posterior vagina to the anterior uterus (where the sperm storage organs are located), and (2) that contracted or closed uterine morphologies occurred before more open morphologies (these were consistent overall with our temporal observations). Thus, using uterine morphology and the amount of ejaculate visible in the vagina and uterus as a guide, we ordered the stages starting from highly contracted virgin female uteruses (Stage 1, see below), to the full, turgid uteruses of mated females in which the sperm storage organs were filled with sperm (Stage 9). A final stage (Stage 10), in which the sperm mass appeared depleted, was observed in uteruses approaching 70min ASM. Using the flow diagram shown in Fig. 3, we were able to quickly and efficiently classify each uterus into its stage. To confirm that we had correctly identified the sperm mass in mated females, and to observe the earliest stages of entry of sperm into storage, we carried out parallel experiments with Oregon R males carrying the dj-GFP transgene (Santel et al., 1997). This allowed us to identify sperm by direct visualization of GFP fluorescence. Crosses between Oregon R females and dj-GFP males did not differ significantly from Oregon R/Oregon R crosses (χ2 = 4.8209, df = 2, Ndj-GFP = 36, NOregon R = 143, p = 0.09; Figs. 4A, B).
Figure 3.
Flow diagram used for classifying digital images of the uterus and vagina of mated females into one of 10 stages of sperm storage. Images were first classified as either “Contracted (curved)” or “Open (straight)”. “Contracted” and “curved” refer to the general conformation of the lower reproductive tract (i.e. as seen in Stages 1–4). In these early stages, substantial contraction of the circular and longitudinal muscles surrounding the uterus occurs, causing folding of the uterus and minimizing the available space within the uterus lumen. The term “Open” refers to the fact that, by Stage 5, the uterus lumen has opened into a single contiguous space. In the latter stages of sperm storage (5–10), the uterus is “straight,” indicating that a straight line can be drawn from the seminal receptacle, through the uterus lumen, to the vagina. Once the images were classified into either “Contracted” or “Open”, we continued down the flow diagram answering yes/no questions until arriving at the appropriate stage classification.
Figure 4.
Progression of stages of morphological change and sperm movement in the female reproductive tract during and after mating (Stages 1–10). Oregon R females were mated once to males that delivered different combinations of accessory gland proteins (Acps) and sperm: (A) Oregon R males (black diamonds) deliver wild-type amounts of both Acps and sperm; (B) dj-GFP males (grey circles) deliver wild-type amounts of Acps and GFP-labeled sperm; (C) Son of tudor males (SOT; grey triangles) deliver wild-type amounts of Acps, but no sperm; (D) DTA-D males (X's superimposed on grey squares) deliver reduced amounts of Acps and sperm; (E) prd-rescue males (+'s superimposed on grey squares) deliver sperm but no Acps; (F) DTA-E males (*'s superimposed on grey squares) deliver neither Acps nor sperm. (B-F) Each pairwise comparison tested the null hypothesis that the coefficient of the interaction term was zero (ASM*Genotype; see text for details). (D-F) The thickened line at Stage 2 serves as a reference when comparing results from mates of DTA-D, prd-rescue, and DTA-E males. The length of the X- and Y-axes vary among panels to reflect the observation times used in the statistical analyses (individual pairwise comparisons were conducted only at times ASM for which data were available from both lines, see text for details). Data from Oregon R Stages 7–10 are grouped into Stage 7 in panel C to match the SOT data (mates of SOT males could not be scored beyond Stage 7 since these males to not produce sperm). P-values are as described in the text; NS = non-significant.
The first 7 stages of sperm storage proceeded quickly (Fig. 4A), and by 10 minutes ASM (in our sample, mating lasted an average of 17.4 ± 0.82 min ; mean ± SE), most of the changes in uterine shape had taken place. With the exception of the stages 2 and 3, we found that overall there was a great deal of variability in the time ASM at which we observed each stage. Furthermore, the timing of stages 4–7 was less consistent (i.e. was skewed) when compared to the remaining stages (also reflected in differences seen between the average and median timing of each stage, values shown below), perhaps due to failed, or less successful, male, female, or interaction effects on mating and sperm storage during this intermediate period. In Stage 4, for example, three extreme values (18, 20, 50 min ASM) had a large influence on the average timing of this stage (14.71 ± 6.49 min ASM (mean ± SE)), even though every other data point took place within 5 min ASM. Nevertheless, we saw no reasonable justification for excluding these variable points from stages 4–7, especially since they likely represent normal levels of variation in the timing of sperm storage in D. melanogaster. Instead (as stated previously), we relied on female lower reproductive tract morphology and sperm accumulation and position when ordering the stages.
Stage 1 (virgin females)
The vagina and uterus (Ut) are compactly arranged into an S-shaped form with the seminal receptacle (SR) and the crescent-shaped “specialized vaginal intima” (SVI, Miller, 1950; actually a part of the posterior uterus; SVI is marked with a dotted line) pulled toward each other ventrally, and the vagina (between SVI and open arrowhead) oriented posterior-dorsally. The walls of the uterus are pressed together. Uterine constriction in this and in subsequent stages appears to be mediated by discrete bands of circular muscles along the length of the anterior and mid-uterus (Miller, 1950; and data not shown). Nevertheless, a small space is sometimes visible in the lumen of the anterior uterus just below the seminal receptacle (not shown). No substances are visible within the lumen of the uterus or vagina. In the posterior uterus, the SVI, vulvar cuticula (Miller, 1950), abdominal tergite 8, and the gonopod are autofluorescent (520nm, CFP filter) under ultraviolet illumination (not shown), and provide important reference points for this and later stages. Although the above description of this stage is based on dissections of virgin females (N=10), during our experimental crosses, 3 females failed to advance past Stage 1, even after mating. On average, these 3 females were frozen 37.67 ± 17.57 min ASM (mean ± SE); median = 50 min ASM). (Filled arrowhead, where the spermathecal ducts open into the uterus; St, spermatheca.)
Stage 2
The vagina and uterus maintain their S-shaped form, although a slight straightening of the mid-uterus (Ut) is sometimes observed. At the anterior uterus, the seminal receptacle (SR) is tightly contracted and pulled slightly posterior along the ventral side of the uterus. The relative positions of several anatomical features (description follows) are important for differentiating this stage from stage 3. An imaginary line run from the center of the seminal receptacle down through the middle of the uterus (longitudinal axis) is parallel to the SVI (adjacent to dotted line). When this longitudinal axis is positioned vertically within the microscope's field of view, the anus (An) is seen at or just posterior (horizontally) to the level of the SVI. In this stage, as well as in stage 3, the anterior uterus is frequently bent laterally in either direction just above the SVI (see also Fig. 1A). Ejaculate (Ej) transfer is visible within the vagina and adjacent to the SVI under visible light. Under UV illumination, small fluorescent masses (within the ejaculate) are also observed within the vaginal lumen and on the inner surface of the SVI (not shown). These masses likely are ejaculatory bulb proteins, which are among the first ejaculatory proteins to enter the female reproductive tract and form the autofluorescent part of the posterior mating plug (Lung and Wolfner, 2001). On average, we observed this stage in females frozen 4.55 ± 0.48 min ASM (mean ± SE); median = 4 min ASM. (CO, common oviduct; filled arrowhead, where the spermathecal ducts open into the uterus; open triangle, points to the gonopod long bristle which is located near the dorsal edge of the vulva.)
Stage 3
The vagina and uterus (Ut) take on an L-shape. The uterus directly above the SVI (dotted line) straightens, and the length of the anterior and mid-uterus are pulled dorsally within the female's abdomen. Longitudinal muscles, which attach at the anterior/mid-uterus and the exoskeleton near the anus (An; at abdominal tergite 8, Miller, 1950; and data not shown), likely produce the dorsal movement of the anterior and mid-uterus. Sperm are present at the anteriormost edge of the ejaculate and, rarely, in a thin longitudinal column inside the constricted mid-uterus (the latter observations were confirmed in females mated to dj-GFP males). On average, we observed this stage in females frozen 4.33 ± 0.53 min ASM (mean ± SE); median = 4 min ASM. (Filled arrowhead, where the spermathecal ducts open into the uterus; open triangle, points to the gonopod long bristle which is located near the dorsal edge of the vulva; PMP, posterior mating plug; SR, seminal receptacle.)
Stage 4
The posterior uterus balloons open above and below the last remaining mid-uterine constriction. A clear fluid of unknown origin is visible in the lumen of the anterior uterus and surrounding the sperm-containing ejaculate in the posterior uterus. This fluid may mediate the expansion of the uterus lumen and is visible in this and all subsequent stages. The seminal receptacle (SR) is no longer shifted ventrally and now sits squarely atop the anterior uterus. Sperm are sometimes seen curved in a thin layer around the inner perimeter of the anterior uterus or in a column within the mid-uterus. Nevertheless, the majority of sperm appear trapped below the constriction in mid-uterus (on one occasion, we observed sperm entering the seminal receptacle during this stage). On average, we observed this stage in females frozen 14.71 ± 6.49 min ASM (mean ± SE); median = 5 min ASM. (An, anus; dotted line, specialized vaginal intima (Miller, 1950); Ej, ejaculate; filled arrowhead, where the spermathecal ducts open into the uterus; open triangle, points to the gonopod long bristle which is located near the dorsal edge of the vulva; PMP, posterior mating plug; Ut, uterus.)
Stage 5
The seminal receptacle (SR), uterus, and vagina are arranged in an almost straight line, although the anterior uterus is usually bent slightly dorsally. The anterior uterus lumen is rounded and open above the hourglass-shaped mid-uterus. A small ball (B) of sperm is often suspended in the anterior uterus. A thin column (C) of sperm in the mid-uterus lies between sperm in the anterior and posterior uterus, the latter of which is filled with ejaculate (Ej). Our fluorescence microscopy observations of green-fluorescent sperm in mates of dj-GFP males (Santel et al., 1997) confirmed our light microscopy observations that sperm within the ball and ejaculate in the posterior uterus appear to be organized into multiple small, doughnut-shaped tori (data not shown). Separate observations of females that were not frozen after mating revealed that these tori slowly rotate. The majority of sperm in this and all subsequent stages were seen in such tori. However, sperm in the column, visible within the constricted mid-uterus, were extended and linear. As of stage 5, we began occasionally to see reproductive tracts in which the seminal receptacle was becoming opaque as it was filling with sperm. On average, we observed this stage in females frozen 10.69 ± 2.57 min ASM (mean ± SE); median = 7 min ASM. (Dotted line, specialized vaginal intima (Miller, 1950); filled arrowhead, where the spermathecal ducts open into the uterus; open triangle, points to the gonopod long bristle which is located near the dorsal edge of the vulva; PMP, posterior mating plug.)
Stage 6
The uterus (Ut) and vagina are aligned linearly and the uterus takes on a turgid, oval shape. The mid-uterus constriction is fully open and the ventral wall of the mid-uterus is curved outward, away from the uterus lumen. The sperm ball (B) is often visible in the anterior uterus (the vagina is out of view in this and in stages 7, 9 and 10). The oviduct valve flap (OVF; which is folded together with and thus indistinguishable from the anterior uterus projection) abuts the low papillate elevation (PE; Miller, 1950), likely blocking passage between the anterior uterus and spermathecal ducts (base of the ducts is denoted by a filled arrowhead) and the common oviduct. On average, we observed this stage in females frozen 9.89 ± 1.87 min ASM (mean ± SE); median = 9 min ASM. (Dotted line, specialized vaginal intima (Miller, 1950); Ej, ejaculate; SR, seminal receptacle.)
Stage 7
The oviduct valve flap (OVF) slides anteriorly (away from the PE, papillate elevation) as the pre-oviduct space (POS) opens and takes on a rectangular shape (in the early part of this stage the POS may not be fully open). Late in Stage 7, which is characterized by a fully open POS, the oviduct valve (OV) and flap are clearly visible. Sperm (in tori) are distributed homogenously throughout the ejaculate, excluding the posterior mating plug (PMP), and often are seen congregating at the uterus walls in the uterus dome (UD). Ejaculate (Ej) was not observed entering the POS. On average, we observed this stage in females frozen 14 ± 1.38 min ASM (mean ± SE); median = 10 min ASM. (Dotted line, specialized vaginal intima (Miller, 1950); filled arrowhead, where the spermathecal ducts open into the uterus; SR, seminal receptacle; *, marks ridge in dorsal oviduct valve.)
Stage 8
The uterus maintains the morphology seen in late Stage 7. The defining characteristic of this stage is a slight lightening or clearing visible in mid-uterus. As sperm (Sp) begin to move through the ejaculate toward the anterior uterus, the anterior mating plug (AMP; which is composed of Acps and is less opaque under visible light than sperm) is left behind, forming a clearer area between the forming sperm mass in the anterior uterus and the PMP. In a few cases, we observed the first sperm entering the spermathecal ducts (filled arrowhead) late in this stage. On average, we observed this stage in females frozen 19.68 ± 1.52 min ASM (mean ± SE); median = 20 min ASM. (Dotted line, specialized vaginal intima (Miller, 1950); open triangle, points to the gonopod long bristle which is located near the dorsal edge of the vulva; SR, seminal receptacle.)
Stage 9
The uterus is fully expanded and turgid. A darkened and discrete sperm mass (SpM) has formed in the anterior uterus and the seminal receptacle (SR) is shaded and full of sperm. The spermathecae are filling with sperm (verified using dj-GFP labeled sperm; openings to ducts denoted by filled arrowhead) and the POS (not visible) is still open and clear of sperm. The entire sperm mass, but not the anterior (AMP) or posterior mating plugs, occasionally appears to have been pushed into the lower common oviduct if the ovaries are detached from the common oviduct, suggesting that the uterine lumen is under a great deal of pressure (see also, Bertram et al., 1996; movement of the ejaculate within the uterus lumen, or exiting the oviduct during or after dissection, was not observed in any other stage). The diameter of the anterior uterus frequently appears wider than the posterior uterus, giving the uterus a slight V-shape. On average, we observed this stage in females frozen 35.65 ± 2.67 min ASM (mean ± SE); median = 36.5 min ASM. (Dotted line, specialized vaginal intima (Miller, 1950).)
Stage 10
The uterus often loses some of its turgidity (vs. Stage 9) and a slight curve on the dorsal side of the POS (pre-oviduct space) is sometime visible. The POS is reduced in size. The seminal receptacle (SR) and spermathecae are full of sperm. The sperm mass (SpM) appears depleted, with space visible between the sperm mass and the uterine walls. On average, we observed this stage in females frozen 43.83 ± 2.66 min ASM (mean ± SE); median = 45 min ASM. (AMP, anterior mating plug; CO, common oviduct; dotted line, specialized vaginal intima (Miller, 1950); filled arrowhead, where the spermathecal ducts open into the uterus; PMP, posterior mating plug.)
3.2. Sperm are not needed for changes in uterus morphology in mated females
Son of tudor (SOT) males lack germline cells and do not produce sperm (Boswell and Mahowald, 1985), but make and transfer Acps and ejaculatory duct and bulb products (Kalb et al., 1993; Tram and Wolfner, 1999). To determine whether sperm are necessary for induction of the reproductive tract changes associated with wild-type sperm storage, we mated SOT males to Oregon R females and, using the series of stages described above, scored female lower reproductive tract morphology at various times up to 50min ASM (Fig. 4C). We used multinomial regression to compare SOT and Oregon R crosses and found that there were no differences (stage as a function of time) between Oregon R females mated to either SOT or Oregon R males (χ2 = 18.07, df = 12, NSOT = 29, NOregon R = 132, p = 0.11). When compared to wild-type Oregon R crosses, the only visible difference in the uteruses of females mated to SOT males was the lack of sperm (Fig. 5). The amount of non-sperm ejaculate, the size of the mating plug, and the overall shape of the uteruses seen in mates of SOT males were almost identical to those in control matings (Fig. 5; and data not shown).
Figure 5.
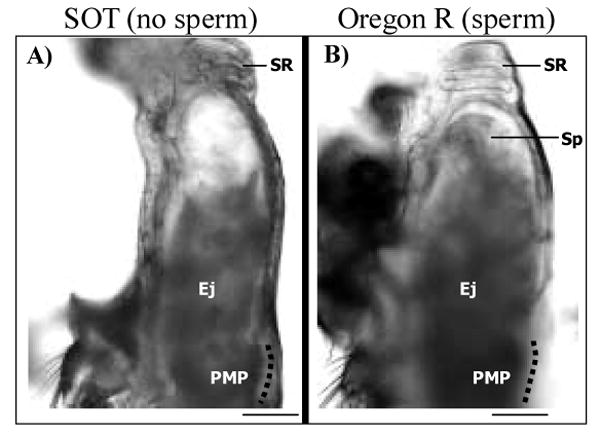
The uteruses of females mated to either Son of Tudor (SOT; A) or Oregon R (B) males look similar. Shown are examples from Stage 6. Since SOT males do not transfer sperm, the volume of the ejaculate (Ej) is noticeably reduced in the uterus in panel A as compared to that of mates of Oregon R males (panel B). Nevertheless, the overall shape of the uterus is the same in mates of both SOT and Oregon R males. The position of the seminal receptacle (SR)and posterior mating plug (PMP), as well as the presence of a clear fluid in the lumen of the uterus (see text for details) does not differ among mates of control or spermless males. Sp, sperm; dotted line parallels the specialized vaginal intima (Miller 1950; Scale = 0.01mm).
3.3. Acps are required for morphological changes during sperm storage in the mated female reproductive tract
Acps are required for wild-type sperm storage (Kalb et al., 1993; Tram and Wolfner, 1999) and utilization (Xue and Noll, 2000). We used three types of male that lack Acps (DTA-E and prd-rescue; Kalb et al., 1993; Xue and Noll, 2000) or produce reduced amounts of Acps (DTA-D; Kalb et al., 1993) to test the hypothesis that wild-type levels of Acps are needed for the morphological changes in the uterus and vagina during sperm storage. All three types of transgenic males transferred ejaculatory bulb proteins, as evidenced by autofluorescence of the protein PEB-me (Lung and Wolfner, 2001) in the posterior mating plug of mated females (data not shown), although posterior mating plug size was reduced in mates of DTA-E and prd-rescue males. Importantly, despite obvious differences between the ejaculates transferred by transgenic versus Oregon R males, the appearance of the uterine and vaginal morphology at each stage (i.e. Stages 1–4) was the same. We found that the odds of Oregon R females mated to DTA-D, prd-rescue, or DTA-E males reaching later stages of sperm storage were significantly lower than crosses between Oregon R pairs (DTA-D vs. Oregon R: χ2 = 155.52, df=18, NDTA-D = 32, NOregon R = 166, p < 0.0001; prd-rescue vs. Oregon R: χ2 = 154.99, df=18, Nprd-rescue = 28, NOregon R = 154, p < 0.0001; DTA-E vs. Oregon R: χ2 = 186.88, df=18, NDTA-E = 39, NOregon R = 166, p < 0.0001; Fig. 4D–F). Within one hour of the start of mating, females mated to DTA-D males had progressed only to stage 4. Mates of prd-rescue and DTA-E males only progressed to stage 3 (except for one of 39 DTA-E-mated female who reached stage 4). In contrast, the majority of females mated to Oregon R males reached the last two stages, 9 and 10, well before 25min ASM. We conducted pairwise comparisons among females mated to males from the three transgenic lines and found that the odds of DTA-D-mated females reaching later stages of sperm storage were significantly higher than the mates of both prd-rescue and DTA-E males (prd-rescue vs. DTA-D: (χ2 = 14.69, df=2, Nprd-rescue = 28, NDTA-D = 32, p < 0.001; DTA-E vs. DTA-D: χ2 = 30.65, df=2, NDTA-E = 39, NDTA-D = 32, p < 0.0001). There was no difference between mates of DTA-E and prd-rescue males (DTA-E vs prd-rescue: χ2 = 2.68, df=2, NDTA-E = 39, Nprd-rescue = 28, p = 0.2617). Thus, morphological changes in females receiving even minimal amounts of Acps from DTA-D males (<1% of wild-type) progressed one stage further (stage 4) during sperm storage than did females that received no Acps. As expected, neither seminal fluid nor sperm were visible in dissections of females mated to DTA-E males, only sperm were visible in dissections of mates of prd-rescue males, and both sperm and seminal fluid were visible in females mated to DTA-D males.
4. Discussion
Sperm introduced into mated females must reach sites of storage for efficient and long-term utilization. In insects, this process has been shown to require input from both females and males. For example, in Tribolium castaneum flour beetles (Bloch Qazi et al., 1998; Fedina and Lewis, 2004) the ability of the female to undergo muscle contraction is necessary for sperm to be stored. In Drosophila melanogaster, a feminized nervous system is necessary for proper accumulation of sperm into storage (Arthur et al., 1998) and male-derived seminal proteins are needed for sperm storage and utilization in the female (Kalb et al., 1993; Tram and Wolfner, 1999; Xue and Noll, 2000). Studies of sperm competition and female sperm preference have also shown roles for both the female and male in sperm-related events. For example, in yellow dung flies (Scatophaga stercoraria) a role has been demonstrated for the female in these processes (Hosken et al., 1999; Parker et al., 1999; Hosken and Ward, 2000), and roles for both sexes in sperm competition and sperm displacement have been found in genetic studies in D. melanogaster (Clark et al., 1995; Clark and Begun, 1998; Clark et al., 1999; Fiumera et al., 2005; Lawniczak and Begun, 2005). Roles for the female can include neural control and muscle contraction to regulate sperm movement into storage (Arthur et al., 1998, Bloch Qazi et al., 1998), fluid absorption by sperm storage organs that suck sperm into storage (in Culex mosquitoes; Linley and Simmons, 1981), and production of secretions that are thought to support sperm storage (e.g. spermathecal secretions in Apis mellifera honeybees; Weirich et al., 2002; Collins et al., 2004).
We report here that in D. melanogaster, a set of dynamic and stereotyped changes occur in the morphology of the female's lower reproductive tract during mating and sperm storage. We categorized these changes into a series of stages, beginning during the transfer of ejaculate by the male. In sum, the uterus and vagina, which are compactly folded within the abdomen of virgin females, enlarge and become turgid during mating. Contraction/relaxation of circular and longitudinal muscles lining the uterus is modulated during insemination and subsequent times during sperm storage; these likely cause most changes we see in shape or position of regions of the lower reproductive tract. Because these morphological changes occur in matings with normal males, but not in males deficient for Acp seminal proteins (but that do transfer non-Acp seminal proteins and sperm), our data indicate that the changes are a response to Acps, and are not simply a passive rearrangement due to the force generated by the male reproductive tract and ejaculate during mating. Females are active participants in the process of sperm storage, a contention that is supported by the results of Arthur et al. (1998) who showed that the female nervous system may actively reduce males' attempts to “force” sperm into the ventral receptacle (called seminal receptacle here), and yet is needed to facilitate the entry of sperm into the spermathecae.
Shifts in the position of several structures in the anterior uterus (including the newly-described oviduct valve flap) can potentially regulate the movement of ejaculate within the anterior uterus, and the accessibility of the sperm-storage organs to the entry of sperm. Prior to stage 7, when the pre-oviduct space opens, the OVF (and AUP) covers the openings to the common oviduct and spermathecal ducts (and likely the parovaria). It is possible that the OVF in this position may block entry by sperm into the oviduct and spermathecal ducts and prevent ejaculate from being mislocalized and forced up into the oviduct during ejaculate transfer. In addition, the subsequent shift in position of the OVF could control the entry of sperm into the spermathecae. Two of our observations are consistent with this idea. First, we did not observe GFP-sperm (from dj-GFP males) in the spermathecal ducts until late stage 8 and stage 9 (similar to the timing observed by Nonidez (1920); data not shown) when the POS is at its most open and the OVF no longer covers the duct openings. Second, in the few Oregon R and dj-GFP crosses that stalled at early stages of sperm storage (i.e. apparent outliers in Stages 5–7 of Figs. 3A, B), we often saw that the seminal receptacle, but not into the spermathecae, was filling with sperm, suggesting that the OVF blocked the opening to the spermathecal ducts.
Our data also suggest several roles for the changes in the region around the specialized vaginal intima (described by Miller, 1950). In the earliest stages (Stages 1–4), uterine circular muscles located just anterior to the SVI appear to regulate the opening of the anterior uterus lumen to allow ejaculate to move toward the sperm storage organs. In addition, the SVI appears to be the site of formation or attachment of the posterior mating plug in the posterior uterus (Stages 2, 3). The morphology of the uterus in Stages 2 and 3 seems to hold ejaculate in contact with the SVI. The shape of the solidified posterior mating plug seen later (Stages 4–10) is the same as the shape of uterus and ejaculate at the SVI in Stages 2 and 3. This suggests that the mating plug may coagulate, or begin to coagulate, during Stages 2 and 3. This is in agreement with the timing of posterior mating plug coagulation reported by Lung and Wolfner (2001). It is also possible that by holding ejaculate in this region of the reproductive tract, the SVI might regulate the time available for Acps to cross the posterior vaginal intima to gain access to the hemolymph (Lung and Wolfner, 1999; Ravi Ram et al., 2005).
By comparing the stages of morphological change reached in matings of wild type females to males that lack Acp seminal proteins, sperm, or both, we found that Acps are necessary to trigger these morphological changes in the lower reproductive tract of the female. In the absence of Acp seminal proteins, the mated female's reproductive tract is unable to proceed beyond Stage 3 (in 2.6% of cases, to Stage 4). Thus, the act of mating, and non-Acp/non-sperm stimuli, is not sufficient to induce the major changes in female reproductive tract conformation (i.e. beyond stage 3); instead, Acps are required for these changes. The inherent variability we observed in wild type Oregon R crosses in the timing of each stage after stage 3 (primarily during stages 4–7) lends support to the contention that this period of time, when the bulk of ejaculate/Acp transfer occurs, may be particularly sensitive to variation in male or female (or both) effects on mating and sperm storage.
Acps have been previously shown to be essential for efficient sperm storage (Kalb et al., 1993; Tram and Wolfner, 1999), but the mechanism by which they accomplished this was unknown. The findings reported here favor a model in which Acps trigger muscle contractions/relaxations in the female's lower reproductive tract that may facilitate the movement of the sperm mass towards the site of storage, and may also expose the openings of those sites. A previous study (Heifetz and Wolfner, 2004) reported that Acps modulate vesicle release at nerve termini in the lower reproductive tract, during the time we examined here. Such regulated release of neuromodulators could mediate muscle contraction/relaxation, causing the morphological changes we report here. It is possible that Acps could participate in other phenomena we observed, such as potentially contributing to the clear liquid in the anterior uterus in Stages 4–10, or in inducing the synthesis or secretion of this fluid (for example, by the parovaria (in Drosophila: Nonidez, 1920; antibacterial activity in Ceratitis capitata,Marchini et al., 1993), spermathecae or spermathecal glands (in Drosophila and Rhodnius prolixus: e.g. Anderson, 1945; Davey and Webster, 1967; Filosi and Perotti, 1975), or uterine lining (in Drosophila: Middleton et al., 2006)). However, available strains, reagents and methods for Drosophila do not allow us to test this possibility.
Curiously, we found that sperm appear to play no role in triggering the shape changes in the lower reproductive tract of mated females: the shape changes occur whether or not females receive sperm. Thus, neither the physical presence of sperm, nor the binding of Acps to sperm (Bertram et al., 1996; Peng et al., 2005) are necessary for the morphological changes in the reproductive tract. Our findings on mates of SOT males also suggest that any differences in female reproductive tract morphology responses to DTA-E males (do not deliver sperm to the female), versus prd-rescue and DTA-D males (which do transfer sperm), are not due to variations in sperm transfer or from any failed sperm-by-Acp interactions.
The failure of sperm to trigger the morphological changes in the lower reproductive tract of the mated female that may facilitate sperm storage leads to the question: to what extent do Drosophila sperm play any active role in their own storage? This is particularly intriguing given the long length of Drosophila sperm and the relatively tight confines of the female reproductive tract (De Vries, 1964; Jamieson, 1987; Pitnick et al., 1995; Miller and Pitnick, 2002). While it seems likely that the muscle contractions and morphological changes documented here aid in the movement of the sperm mass to the site of entry into storage, this does not mean that sperm need be passive participants in their storage. Sperm are motile upon transfer to the female (Nonidez, 1920; Anderson, 1945; DeVries, 1964; E.M.A., unpublished observations), and we observed differences in the extension of the sperm mass and between groups of sperm during storage. In much of the lower female reproductive tract, sperm are found in multiple toroidal bundles. When they pass through a constricted space (e.g. “column” (C) between anterior and posterior uterus, Fig. 2, Stage 5; and inside female sperm storage organs, Pitnick et al., 1995;Miller and Pitnick, 2002), they appear elongated. Whether this involves any active movement by the sperm, or is entirely a passive consequence of the environment (shape, pressure due to muscle contraction) is unknown. However, since a small number of sperm do get stored in Acp-less matings (Tram and Wolfner, 1999; Xue and Noll, 2000), the morphological changes we report here, while likely responsible for the bulk of sperm storage, are not absolutely required for a sperm to be able to enter storage. This is also consistent with the observation noted above that we sometimes saw sperm entering the seminal receptacle in mates of Oregon R or dj-GFP males in which morphological changes had stalled at early stages.
In summary, we found that sperm transfer and storage in D. melanogaster is a dynamic process in which both males and females play active roles. During mating, the lower female reproductive tract (vagina and uterus) begins a series of 10 stereotyped stages, starting from a highly contracted virgin morphology (Stage 1), to expanded mated stages (Stage 6–9), and ending with a depleted final stage (10) in which a large proportion of sperm transferred into the uterus has been stored in the seminal receptacle and spermathecae. Acps from the male are required to elicit the changes, which appear to be largely muscular in nature. The intrinsic nature of the morphological changes occurring within the female suggest that females play an active role in sperm storage. In the absence of Acps, mates of DTA-E and prd-rescue males do not progress beyond Stage 3 of morphological changes, suggesting that Acps provide important cues needed for continued sperm transfer and storage. Receipt of even minimal amounts of Acps (<1%; DTA-D males) allows for the progression to Stage 4, when considerable expansion of the uterus lumen begins. Without sperm, or the binding of sperm to Acps, the stages progress normally. In conclusion, our results suggest that female muscular control and Acps (but not sperm) from the male interact to mediate changes in female morphology needed for successful sperm storage.
Acknowledgments
We thank M. Bloch Qazi, N. Buehner, V. Horner, S. Ji, K. Latham, L. McGraw, J. Mueller, K, Ravi Ram, K. Sackton, L. Sirot, A. Wong for helpful assistance, advice, suggestions and/or comments on this manuscript, and two J.I.P reviewers for helpful suggestions. We thank B. Wakimoto and M. Noll for dj-GFP and prd-rescue flies, respectively, and A. Yori for the artwork in Fig. 1. This work was funded by NIH grant HD38921 to MFW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonso-Pimentel H, Tolbert LP, Heed WB. Ultrastructural examination of the insemination reaction in Drosophila. Cell and Tissue Research. 1994;275:467 –479. doi: 10.1007/BF00318816. [DOI] [PubMed] [Google Scholar]
- Anderson RC. A study of the factors affecting fertility of lozenge females of Drosophila melanogaster. Genetics. 1945;30:280–296. doi: 10.1093/genetics/30.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur BI, Jr, Hauschteck-Jungen E, Nöthiger R, Ward PI. A female nervous system is necessary for normal sperm storage in Drosophila melanogaster: a masculinized nervous system is as good as none. Proceedings B of the Royal Society of London. 1998;265:1749–1753. [Google Scholar]
- Bairati A. Structure and ultrastructure of the male reproductive system in Drosophila melanogaster Meig. 2 The genital duct and accessory glands. Italian Journal of Zoology. 1968;2:105–182. [Google Scholar]
- Bairati A, Perotti ME. Occurence of a compact plug in the genital duct of Drosophila females after mating. Drosophila Information Service. 1970;45:67–68. [Google Scholar]
- Bertram MJ, Neubaum DM, Wolfner MF. Localization of the Drosophila male accessory gland protein Acp36DE in the mated female suggests a role in sperm storage. Insect Biochemistry and Molecular Biology. 1996;26:971–980. doi: 10.1016/s0965-1748(96)00064-1. [DOI] [PubMed] [Google Scholar]
- Birkhead TR. Cryptic female choice: criteria for establishing female sperm choice. Evolution. 1998;52:1212–1218. doi: 10.1111/j.1558-5646.1998.tb01848.x. [DOI] [PubMed] [Google Scholar]
- Birkhead TR, Møller AP. Female control of paternity. Trends in Ecology and Evolution. 1993;8:100–104. doi: 10.1016/0169-5347(93)90060-3. [DOI] [PubMed] [Google Scholar]
- Bloch Qazi MC, Aprille JR, Lewis SM. Female role in sperm storage in the red flour beetle, Tribolium castaneum. Comparative Biochemistry and Physiology A. 1998;120:641 –647. [Google Scholar]
- Bloch Qazi MC, Wolfner MF. An early role for the Drosophila melanogaster male seminal protein Acp36DE in female sperm storage. Journal of Experimental Biology. 2003;206:3521–3528. doi: 10.1242/jeb.00585. [DOI] [PubMed] [Google Scholar]
- Bloch Qazi MC, Heifetz Y, Wolfner MF. The developments between gametogenesis and fertilization: ovulation and female sperm storage in Drosophila melanogaster. Developmental Biology. 2003;256:195–211. doi: 10.1016/s0012-1606(02)00125-2. [DOI] [PubMed] [Google Scholar]
- Boswell RE, Mahowald AP. tudor, a gene required for assembly of the germ plasm in Drosophila melanogaster. Cell. 1985;43:97–104. doi: 10.1016/0092-8674(85)90015-7. [DOI] [PubMed] [Google Scholar]
- Bryant PJ. Pattern formation in imaginal discs. In: Ashburner M, Wright TRF, editors. The Genetics and Biology of Drosophila. 2C. Academic Press; NY: 1978. pp. 230–336. [Google Scholar]
- Clark AG, Aguade M, Prout T, Harshman LG, Langley CH. Variation in sperm displacement and its association with accessory gland protein loci in Drosophila melanogaster. Genetics. 1995;139:189–201. doi: 10.1093/genetics/139.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AG, Begun DJ. Female genotypes affect sperm displacement in Drosophila. Genetics. 1998;149:1487–93. doi: 10.1093/genetics/149.3.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AG, Begun DJ, Prout T. Female x male interactions in Drosophila sperm competition. Science. 1999;283:217–20. doi: 10.1126/science.283.5399.217. [DOI] [PubMed] [Google Scholar]
- Collins AM, Williams V, Evans JD. Sperm storage and antioxidative enzyme expression in the honey bee, Apis mellifera. Insect Molecular Biology. 2004;13:141–146. doi: 10.1111/j.0962-1075.2004.00469.x. [DOI] [PubMed] [Google Scholar]
- Davey KG, Webster GF. Structure and secretion of spermatheca of Rhodnius prolixus Stal - a histochemical study. Canadian Journal of Zoology. 1967;45:653–657. [Google Scholar]
- DeVries JK. Insemination and sperm storage in Drosophila melanogaster. Evolution. 1964;18:271–282. [Google Scholar]
- Eberhard WG. Female Control: Sexual Selection by Cryptic Female Choice. Princeton University Press; Princeton, NJ: 1996. [Google Scholar]
- Fedina TY, Lewis SM. Female influence over offspring paternity in the red flour beetle Tribolium castaneum. Proceedings B of the Royal Society of London. 2004;271:1393–9. doi: 10.1098/rspb.2004.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosi M, Perotti ME. Fine structure of the spermatheca of Drosophila melanogaster. Journal of Submicroscopic Cytology. 1975;7:259–270. [Google Scholar]
- Fiumera AC, Dumont BL, Clark AG. Sperm competitive ability in Drosophila melanogaster associated with variation in male reproductive proteins. Genetics. 2005;169:243–57. doi: 10.1534/genetics.104.032870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler GL. Some aspects of the reproductive biology of Drosophila: sperm transfer, sperm storage, and sperm utilization. Advances in Genetics. 1973;17:293–360. [Google Scholar]
- Fuyama Y. Species specificity of paragonial substances as an isolating mechanism in Drosophila. Experientia. 1983;39:190–192. [Google Scholar]
- Gilbert DG. Ejaculate esterase 6 and initial sperm use by female Drosophila melanogaster. Journal of Insect Physiology. 1981;27:641–650. [Google Scholar]
- Gillot C. Male accessory gland secretions: Modulators of female reproductive physiology and behavior. Annual Review of Entomology. 2003;48:163–184. doi: 10.1146/annurev.ento.48.091801.112657. [DOI] [PubMed] [Google Scholar]
- Gromko MH, Gilbert DG, Richmond RC. Sperm transfer and use in the multiple mating system of Drosophila. In: Smith RL, editor. Sperm Competition and the Evolution of Animal Mating Systems. Academic Press; London: 1984. pp. 371–426. [Google Scholar]
- Heifetz Y, Lung O, Frongillo EA, Jr, Wolfner MF. The Drosophila seminal fluid protein Acp26Aa stimulates release of oocytes by the ovary. Current Biology. 2000;10:99–102. doi: 10.1016/s0960-9822(00)00288-8. [DOI] [PubMed] [Google Scholar]
- Heifetz Y, Wolfner MF. Mating, seminal fluid components, and sperm cause changes in vesicle release in the Drosophila female reproductive tract. Proceedings of the National Academy of Sciences USA. 2004;101:6261–6. doi: 10.1073/pnas.0401337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hihara F. Effects of the male accessory gland secretion on oviposition and remating in females of Drosophila melanogaster. Zoological Magazine. 1981;90:307 –316. [Google Scholar]
- Hosken DJ, Ward PI. Copula in yellow dung flies (Scathophaga stercoraria): investigating sperm competition models by direct investigation. Journal of Insect Physiology. 2000;46:1355–1363. doi: 10.1016/s0022-1910(00)00057-3. [DOI] [PubMed] [Google Scholar]
- Hosken DJ, Meyer E, Ward PI. Internal female reproductive anatomy and genitalic interactions during copula in the yellow dung fly, Scathophaga stercoraria (Diptera: Scathophagidae) Canadian Journal of Zoology. 1999;77:1975–1983. [Google Scholar]
- Iida K, Cavener DR. Glucose dehydrogenase is required for normal sperm storage and utilization in female Drosophila melanogaster. Journal of Experimental Biology. 2004;207:675–81. doi: 10.1242/jeb.00816. [DOI] [PubMed] [Google Scholar]
- Jamieson BGM. The Ultrastructure and Phylogeny of Insect Spermatozoa. Cambridge University Press; Cambridge, UK: 1987. [Google Scholar]
- Kalb J, DiBenedetto AJ, Wolfner MF. Probing the function of Drosophila melanogaster accessory glands by directed cell ablation. Proceedings of the National Academy of Sciences USA. 1993;90:8093–8097. doi: 10.1073/pnas.90.17.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman BP, Demerec M. Utilization of sperm by the female Drosophila melanogaster. American Naturalist. 1942;76:445–469. [Google Scholar]
- Lawniczak MK, Begun DJ. A QTL analysis of female variation contributing to refractoriness and sperm competition in Drosophila melanogaster. Genetical Research. 2005;86:107–14. doi: 10.1017/S0016672305007755. [DOI] [PubMed] [Google Scholar]
- Lefevre G, Jonsson UB. Sperm transfer, storage, displacement, and utilization in Drosophila melanogaster. Genetics. 1962;47:1719 –1736. doi: 10.1093/genetics/47.12.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linley JR. Emptying of the spermatophore and spermathecal filling in Culicoides melleus (Coq.) (Diptera: Ceratopogonidae) Canadian Journal of Zoology. 1981;59:347 –356. [Google Scholar]
- Liu H, Kubli E. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proceedings of the National Academy of Sciences USA. 2003;100:9929–9933. doi: 10.1073/pnas.1631700100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig MZ, Uspensky II, Ivanov AI, Kopantseva MR, Dianov CM, Tamarina NA, Korochkin LI. Genetic control and expression of the major ejaculatory bulb protein (PEB-me) in Drosophila melanogaster. Biochemical Genetics. 1991;29:215 –239. doi: 10.1007/BF00590103. [DOI] [PubMed] [Google Scholar]
- Lung O, Wolfner MF. Drosophila seminal fluid proteins enter the circulatory system through the walls of the posterior vagina. Insect Biochemistry and Molecular Biology. 1999;29:1043–1052. doi: 10.1016/s0965-1748(99)00078-8. [DOI] [PubMed] [Google Scholar]
- Lung O, Wolfner MF. Identification of and characterization of the major D. melanogaster mating plug protein. Insect Biochemistry and Molecular Biology. 2001;31:543–551. doi: 10.1016/s0965-1748(00)00154-5. [DOI] [PubMed] [Google Scholar]
- Manning A. A sperm factor affecting the receptivity of Drosophila melanogaster females. Nature. 1962;194:252–253. [Google Scholar]
- Manning A. The control of sexual receptivity in female Drosophila. Animal Behavior. 1967;15:239–250. doi: 10.1016/0003-3472(67)90006-1. [DOI] [PubMed] [Google Scholar]
- Marchini D, Giordano PC, Amons R, Bernini LF, Dallai R. Purification and primary structure of ceratotoxin A and B, two antibacterial peptides from the female reproductive accessory glands of the medfly Ceratitis capitata (Insecta:Diptera) Insect Biochemistry and Molecular Biology. 1993;23:591–8. doi: 10.1016/0965-1748(93)90032-n. [DOI] [PubMed] [Google Scholar]
- Meikle DB, Sheehan KB, Phillis DM, Richmond RC. Localization and longevity of seminal-fluid esterase 6 in mated female Drosophila melanogaster. Journal of Insect Physiology. 1990;14:1159–1168. [Google Scholar]
- Middleton CA, Nongthomba U, Parry K, Sweeney ST, Sparrow JC, Elliott CG. Neuromuscular organization and aminergic modulation of contractions in the Drosophila ovary. BMC Biology. 2006;4:17. doi: 10.1186/1741-7007-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. The internal anatomy and histology of the imago of Drosophila melanogaster. In: Demerec M, editor. Biology of Drosophila. Wiley; New York: 1950. pp. 420–534. [Google Scholar]
- Miller GT, Pitnick S. Sperm-female coevolution in Drosophila. Science. 2002;298:1230–3. doi: 10.1126/science.1076968. [DOI] [PubMed] [Google Scholar]
- Monsma SA, Harada HA, Wolfner MF. Synthesis of two Drosophila male accessory gland proteins and their fate after transfer to the female during mating. Developmental Biology. 1990;142:465–475. doi: 10.1016/0012-1606(90)90368-s. [DOI] [PubMed] [Google Scholar]
- Neubaum DM, Wolfner MF. Wise, winsome or wierd: Mechanisms of sperm storage in female animals. Current Topics in Developmental Biology. 1999a;41:67–97. doi: 10.1016/s0070-2153(08)60270-7. [DOI] [PubMed] [Google Scholar]
- Neubaum DM, Wolfner MF. Drosophila females require a seminal fluid protein, Acp36DE, to store sperm efficiently. Genetics. 1999b;153:845–857. doi: 10.1093/genetics/153.2.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonidez JF. The internal phenomena of reproduction in Drosophila. Biological Bulletins of Woods Hole. 1920;39:207–230. [Google Scholar]
- Parker G. Sperm competition and its evolutionary consequences in the insects. Biological Reviews. 1970;45:525–567. [Google Scholar]
- Parker GA, Simmons LW, Stockley P, McChristie DM, Charnov EL. Optimal copula duration in yellow dung flies: effects of female size and egg content. Animal Behavior. 1999;57:795–805. doi: 10.1006/anbe.1998.1034. [DOI] [PubMed] [Google Scholar]
- Patterson JT, Stone WS. Evolution in the genus Drosophila. Macmillan; New York: 1952. [Google Scholar]
- Peng J, Chen S, Busser S, Liu H, Honegger T, Kubli E. Gradual release of sperm bound sex-peptide controls female postmating behavior in Drosophila. Current Biology. 2005;15:207–13. doi: 10.1016/j.cub.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Pitnick S, Spicer GS, Markow TA. How long is a giant sperm? Nature. 1995;375:109. doi: 10.1038/375109a0. [DOI] [PubMed] [Google Scholar]
- Ravi Ram K, Ji S, Wolfner MF. Fates and targets of male accessory gland proteins in mated female Drosophila melanogaster. Insect Biochemistry and Molecular Biology. 2005;35:1059–1071. doi: 10.1016/j.ibmb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Riedel IH, Kruse K, Howard J. A self-organized vortex array of hydrodynamically entrained sperm cells. Science. 2005;309:300–303. doi: 10.1126/science.1110329. [DOI] [PubMed] [Google Scholar]
- Santel A, Winhauer T, Blumer N, Renkawitz-Pohl R. The Drosophila don juan (dj) gene encodes a novel sperm specific protein component characterized by an unusual domain of repetitive amino acid motif. Mechanisms of Development. 1997;64:19–30. doi: 10.1016/s0925-4773(97)00031-2. [DOI] [PubMed] [Google Scholar]
- Thornhill R. Cryptic female choice and its implications in the scorpionfly Harpobittacus nigriceps. American Naturalist. 1983;122:765–788. [Google Scholar]
- Tram U, Wolfner MF. Male seminal fluid proteins are essential for sperm storage in Drosophila melanogaster. Genetics. 1999;153:837–844. doi: 10.1093/genetics/153.2.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward PI. Intraspecific variation in sperm size characters. Heredity. 1998;80:655–699. doi: 10.1046/j.1365-2540.1998.00401.x. [DOI] [PubMed] [Google Scholar]
- Weirich GF, Collins AM, Williams VP. Antioxidant enzymes in the honeybee, Apis mellifera L. Apidologie. 2002;33:3–14. [Google Scholar]
- Xue L, Noll M. Drosophila female sexual behavior induced by sterile males showing copulation complementation. Proceedings of the National Academy of Sciences USA. 2000;97:3272 –3275. doi: 10.1073/pnas.060018897. [DOI] [PMC free article] [PubMed] [Google Scholar]