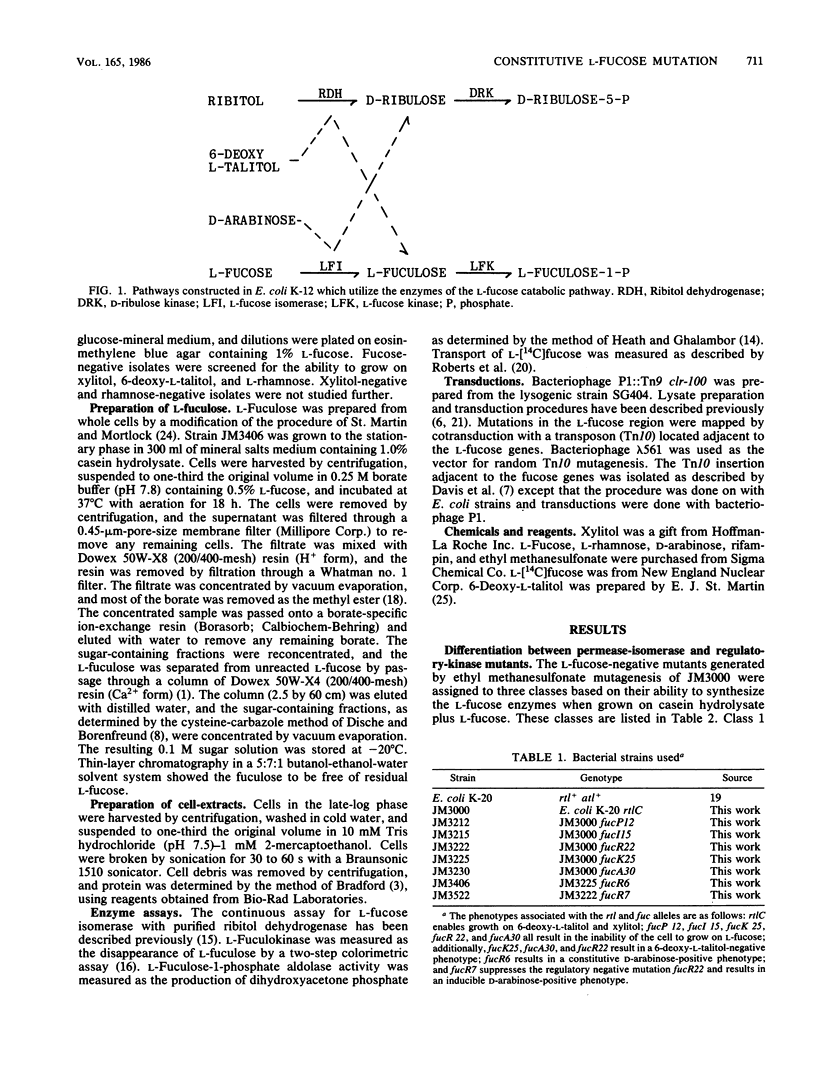
Abstract
A ribitol-positive transductant of Escherichia coli K-12, JM2112, was used to facilitate the isolation and identification of mutations affecting the L-fucose catabolic pathway. Analysis of L-fucose-negative mutants of JM2112 enabled us to confirm that L-fucose-1-phosphate is the apparent inducer of the fucose catabolic enzymes. Plating of an L-fuculokinase-negative mutant of JM2112 on D-arabinose yielded an isolate containing a second fucose mutation which resulted in the constitutive synthesis of L-fucose permease, isomerase, and kinase. This constitutive mutation differs from the constitutive mutation described by Chen et al. (J. Bacteriol. 159:725-729, 1984) in that it is tightly linked to the fucose genes and appears to be located in the gene believed to code for the positive activator of the L-fucose genes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartkus J. M., Mortlock R. P. Construction of an improved D-arabinose pathway in Escherichia coli K-12. J Bacteriol. 1986 Mar;165(3):704–709. doi: 10.1128/jb.165.3.704-709.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chakrabarti T., Chen Y. M., Lin E. C. Clustering of genes for L-fucose dissimilation by Escherichia coli. J Bacteriol. 1984 Mar;157(3):984–986. doi: 10.1128/jb.157.3.984-986.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. M., Chakrabarti T., Lin E. C. Constitutive activation of L-fucose genes by an unlinked mutation in Escherichia coli. J Bacteriol. 1984 Aug;159(2):725–729. doi: 10.1128/jb.159.2.725-729.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISCHE Z., BORENFREUND E. A new spectrophotometric method for the detection and determination of keto sugars and trioses. J Biol Chem. 1951 Oct;192(2):583–587. [PubMed] [Google Scholar]
- EAGON R. G. Bacterial dissimilation of L-fucose and L-rhamnose. J Bacteriol. 1961 Oct;82:548–550. doi: 10.1128/jb.82.4.548-550.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GHALAMBOR M. A., HEATH E. C. The metabolism of L-fucose. II. The enzymatic cleavage of L-fuculose 1-phosphate. J Biol Chem. 1962 Aug;237:2427–2433. [PubMed] [Google Scholar]
- GREEN M., COHEN S. S. Enzymatic conversion of L-fucose to L-fuculose. J Biol Chem. 1956 Apr;219(2):557–568. [PubMed] [Google Scholar]
- HEATH E. C., GHALAMBOR M. A. The metabolism of L-fucose. I. The purification and properties of L-fuculose kinase. J Biol Chem. 1962 Aug;237:2423–2426. [PubMed] [Google Scholar]
- Hacking A. J., Lin E. C. Regulatory changes in the fucose system associated with the evolution of a catabolic pathway for propanediol in Escherichia coli. J Bacteriol. 1977 May;130(2):832–838. doi: 10.1128/jb.130.2.832-838.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. J., Mortlock R. P. Metabolism of D-arabinose: a new pathway in Escherichia coli. J Bacteriol. 1971 Apr;106(1):90–96. doi: 10.1128/jb.106.1.90-96.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R., Cohen S. S. D-phosphoarabinoisomerase and D-ribulokinase in Escherichia coli. J Biol Chem. 1966 Oct 10;241(19):4304–4315. [PubMed] [Google Scholar]
- Reiner A. M. Genes for ribitol and D-arabitol catabolism in Escherichia coli: their loci in C strains and absence in K-12 and B strains. J Bacteriol. 1975 Aug;123(2):530–536. doi: 10.1128/jb.123.2.530-536.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S., Paden C. A., Greenberg E. P. Uptake of D-xylose and D-glucose by Spirochaeta aurantia. J Bacteriol. 1984 Jul;159(1):427–428. doi: 10.1128/jb.159.1.427-428.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint Martin E. J., Mortlock R. P. Natural and altered induction of the L-fucose catabolic enzymes in Klebsiella aerogenes. J Bacteriol. 1976 Jul;127(1):91–97. doi: 10.1128/jb.127.1.91-97.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skjold A. C., Ezekiel D. H. Analysis of lambda insertions in the fucose utilization region of Escherichia coli K-12: use of lambda fuc and lambda argA transducing bacteriophages to partially order the fucose utilization genes. J Bacteriol. 1982 Oct;152(1):120–125. doi: 10.1128/jb.152.1.120-125.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skjold A. C., Ezekiel D. H. Regulation of D-arabinose utilization in Escherichia coli K-12. J Bacteriol. 1982 Oct;152(1):521–523. doi: 10.1128/jb.152.1.521-523.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Martin E. J., Mortlock R. P. Biosynthesis and catabolism of 6-deoxy L-talitol by Klebsiella aerogenes mutants. J Bacteriol. 1980 Mar;141(3):1157–1162. doi: 10.1128/jb.141.3.1157-1162.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]



