Abstract
Catechol-O-methyltransferase (COMT) is one of the major mammalian enzymes involved in the metabolic degradation of catecholamines and is considered a candidate for several psychiatric disorders and symptoms, including the psychopathology associated with the 22q11 microdeletion syndrome. By means of homologous recombination in embryonic stem cells, a strain of mice in which the gene encoding the COMT enzyme has been disrupted was produced. The basal concentrations of brain catecholamines were measured in the striatum, frontal cortex, and hypothalamus of adult male and female mutants. Locomotor activity, anxiety-like behaviors, sensorimotor gating, and aggressive behavior also were analyzed. Mutant mice demonstrated sexually dimorphic and region-specific changes of dopamine levels, notably in the frontal cortex. In addition, homozygous COMT-deficient female (but not male) mice displayed impairment in emotional reactivity in the dark/light exploratory model of anxiety. Furthermore, heterozygous COMT-deficient male mice exhibited increased aggressive behavior. Our results provide conclusive evidence for an important sex- and region-specific contribution of COMT in the maintenance of steady-state levels of catecholamines in the brain and suggest a role for COMT in some aspects of emotional and social behavior in mice.
Catechol-O-methyltransferase (COMT) along with monoamine oxidases (MAO-A and -B) are the major mammalian enzymes involved in the metabolic degradation of dopamine, norepinephrine, and epinephrine. COMT is a Mg2+-dependent enzyme that catalyzes the transfer of methyl groups from S-adenosyl methionine to a hydroxyl group of a catecholic substrate: dopamine is converted into 3-methoxytyramine, and norepinephrine is converted into normetanephrine (1). COMT is widely distributed in the mammalian brain, although results from pharmacological studies suggest that the relative importance of methylation (by COMT) versus deamination (by MAO) in the metabolic degradation of catecholamines varies among brain regions, with methylation accounting for about 15% of released dopamine in striatum and in nucleus accumbens and for more than 60% in frontal cortex (2). The enzyme is absent from the dopaminergic terminals and is thought to be involved in the catabolism of extraneuronal dopamine in glial cells and/or postsynaptic neurons (1). COMT activity is probably under hormonal control. Comparisons of liver COMT activity and thermostability in humans indicated an epigenetically determined significantly lower COMT activity in females (3). A limited number of studies in other species suggest that COMT activity can be reduced epigenetically by estrogens (4) and can be affected by the process of sexual differentiation of the brain (5).
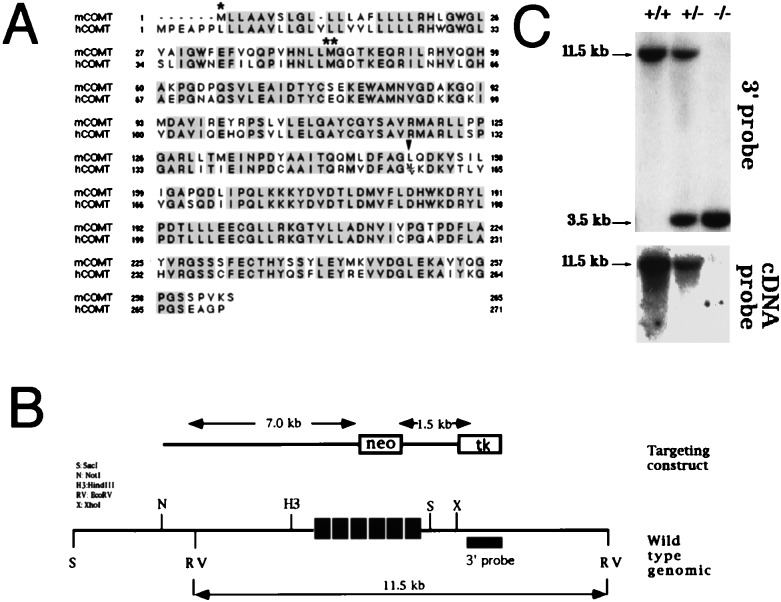
The gene for COMT is located at the q11 band of human chromosome 22, which has been reported previously to be hemizygously deleted in patients with schizophrenia, childhood onset schizophrenia, and obsessive compulsive disorder (OCD) (6, 7). In addition, a high frequency of psychiatric symptoms, including anxiety, depression, and obsessive compulsive symptoms (8), has been described in children and adults with the 22q11 microdeletion. The comt gene therefore, because of its function and its genomic location, represents an attractive candidate gene as a contributor (in a dosage-dependent way) to some of the psychiatric symptoms and disorders associated with the hemizygous 22q11 deletion. In humans, a common polymorphism (a valine to methionine substitution at codon 158 of the gene, Fig. 1A), which causes a 3- to 4-fold variation in the COMT enzyme activity, has been used to address the issue of direct involvement of this gene in susceptibility to psychiatric diseases. Studies on the comt allele distribution, as well as extensive mutational analysis failed to reveal a major effect of low COMT activity on schizophrenia (9), although studies on case/control samples (10) and more recently affected families (M.K., C. A. Sobin, M. L. Blundell, B. L. Galke, L. Malinova, P. Goldberg, J. Oh, and J.A.G., unpublished results) suggested that the low activity allele of this gene is significantly associated with susceptibility to OCD in a recessive and sexually dimorphic manner. In addition, earlier studies assaying enzyme activity suggested a sexually dimorphic role for this gene in affective disorder (11, 12), an observation supported by more recent studies using genetic markers (13). The involvement of COMT in the monoamine metabolic pathways could explain the pleiotropic effect of this gene on susceptibility to psychiatric disorders and symptoms. However, the details of this involvement remain elusive mainly because of the limitations associated with techniques for examining central nervous system function in humans. The availability of a mouse model for a gene considered to be a candidate for a common, complex psychiatric disorder, although unlikely to serve as a model for the entire complexity of the disorder, could provide a framework for understanding the involvement of this gene. In the case of COMT it also could provide insights regarding the basis of the, largely unexpected, sexually dimorphic pattern of associations with psychiatric disorders. To start addressing these issues, we have developed and initially characterized a strain of mice in which the gene encoding the COMT enzyme has been disrupted.
Figure 1.
Targeting of the COMT locus. (A) Sequence alignment of mouse and human COMT. Single and double asterisks indicate the membrane bound and soluble initiation methionines, respectively (by analogy to the rat and human clones). Arrowhead indicates codon 158 of the human gene where a met/val variation determines low or high activity of the enzyme (9, 31, 38). (B) Targeting of mouse COMT locus in embryonic stem cells and mice. A HindIII–SacI fragment was shown to encompass the entire set of coding exons of the COMT gene. For the construction of the targeting construct, part of this fragment, encompassing exons 2–4 (see below) was replaced by a cassette including the neo gene under the control of the phosphoglycerol kinase promoter. Cell culture, electroporation of A7ES cells, and generation of the chimeric mice were performed essentially as previously described (39). About 15% of the tested embryonic stem cell clones were positive for homologous recombination, and three clones were selected for karyotyping and injection into C57B6 blastocysts. Chimeric males were mated with C57B6 females, and DNA from tail biopsies of F1 agouti coat pups was typed by Southern blotting and PCR at the comt genomic locus. F1 heterozygous mice were mated, and F2 mice of all three genotypes and of mixed 129/J/C57B6 background were obtained. Arrow lines indicate the diagnostic EcoRV restriction fragment. The probe used for Southern analysis is shown as a thick black line. (C) Genomic Southern blot analysis of tail biopsies. Genomic DNA was isolated from offspring obtained after breeding of heterozygous mice, digested with EcoRV, and probed with a ≈1-kb fragment adjacent to the right arm of the targeting construct or a 5′ coding portion of the mouse COMT cDNA, corresponding to exons 2–4. In the former case, wild-type and recombinant restriction fragments are 11.5 and 3.5 kb, respectively.
MATERIALS AND METHODS
Neurochemical Measures.
Mice brains were rapidly excised after decapitation and were frozen on powdered dry ice. Coronal sections were made with a razor blade, mounted onto a microscope slide, and stored at −70°C until sampling. Brain areas were micropunched from the sections according to the atlas of Franklin and Paxinos (14) with a 500-mm diameter cannula while the slide rested on the stage of a dissecting microscope that was maintained at −15°C. The frontal cortex section was between approximately +3.08 and +1.70 mm, and four dorsomedial punches were taken. It contained the medial and ventral orbital cortex and the pre- and infra-limbic cortex. Bilateral punches of the dorsal striatum were taken at the level of the optic chiasm, between approximately +.50 mm and −0.22 mm. The hypothalamus was sampled with bilateral punches between approximately −0.34 and −2.18 mm and consisted of the dorso- and ventromedial nuclei, anterior hypothalamic nucleus, and arcuate nucleus. Levels of dopamine and its metabolites homovanillic acid (HVA) and dihydroxyphenylacetic acid (DOPAC), as well as of norepinephrine, 5-hydroxytryptamine, and 5-hydroxy indole acetic acid, were measured by using HPLC with electrochemical detection as described previously (15). The lower limits of detection for monoamines and metabolites were approximately 500 fg, and HVA was detectable to 50 fg. Briefly, punched tissue was expelled into a sodium acetate buffer (pH 5.0) containing 1 × 10−7 M of α-methyl dopamine as an internal standard (120 μl for striatum and 60 μl for the other areas). After freeze-thawing and centrifugation, the supernatant was removed and 2 μl of a 1 mg/ml of ascorbate oxidase solution (Sigma) was added to each sample to minimize the front. Forty microliters was injected in a Waters Associates chromatographic system consisting of a 717 Plus autosampler, 590 pump, and C-18 reverse-phase 3-μm Velosep column (Rainin Instruments). An EsA 50l1 Coulocomb 3100A electrochemical detector with the screening electrode set at +0.05 V and the detecting electrode at +0.35 V was used. Concentrations of neurotransmitters and metabolites were calculated by reference to standards using peak integration with a computer-assisted Waters Millenium system. The pellet was dissolved in 100 μl of 0.2 N NaOH for protein determination by the Bradford method. Concentrations are expressed as pg/μg protein. Measurements were made in two cohorts of mice consisting of male and female homozygous and wild type and pooled for statistical analysis when no sex differences were observed.
Behavioral Measures.
Sixty-four mice of both sexes and all three genotypes, 11–16 weeks old at the onset of testing, were housed individually for 5–6 months with free access to food and water. They were maintained on a reverse 12/12-h light/dark cycle with lights off at 0700 h. All testing occurred between 0900 and 1800 h. Before all testing, animals were handled, weighed, and pre-exposed to the testing chamber.
Locomotor Activity and Anxiety-Like Behaviors.
To minimize the influence of anxiety on spontaneous locomotor activity level, animals were handled and pre-exposed to the chamber before testing, and activity was monitored under indirect dim light and sound-attenuated conditions. Testing took place in a clear acrylic chamber (40.5 × 40.5 × 30 cm) equipped with infrared sensors for the automatic recording of horizontal activity (Digiscan Model RXYZCM, Accuscan Instruments, Columbus, OH). Each subject initially was placed in the center of the chamber, and time spent ambulating and total distance traveled over the next 10 min were used as the measure of activity. After the initial test of locomotor activity, the effect of the mutation on anxiety was recorded in a dark/light exploratory model in a two-compartment light/dark box. The apparatus and conditions were similar to those used above, except that an enclosed black acrylic box (40 × 20.5 × 20.5 cm) was inserted into the right half of the chamber with an opening (13 × 5 cm) allowing for passage between the two compartments monitored by an infrared beam. The open compartment was now directly illuminated by a 60-W bulb placed 40 cm above the floor of the compartment. Animals initially were placed in the center of the dark compartment, and data collection commenced immediately for 10 min. Two tests were performed for the locomotor and anxiety assays, and at the end of the assays the genotypes of the mice were reconfirmed by Southern blot analysis.
Prepulse Inhibition (PPI) of the Startle Response.
PPI was assayed several weeks after the dark/light test (16, 17). Each of two startle chambers (SR-Lab, San Diego Instruments) contained a transparent acrylic cylinder (4 cm in diameter) mounted on a frame to which a motion sensor was attached for the detection and transduction of movement, and a sound generation system was used for the delivery of background white noise and acoustic stimuli. A CompuAdd 386 microprocessor and San Diego Instruments interface board and software were used for the delivery of stimuli and response recording (100 1-ms readings beginning at startle stimulus onset). Response amplitude was calculated as the maximum response level occurring during the 100-ms recording. Both chambers were calibrated for equivalent stimulus intensities and response sensitivities, and experimental groups were balanced across chambers. Immediately after placement in the chamber, the animal was given a 4-min acclimation period during which background noise (65 dB) was continually present, and then received four no-stimulus trials, four startle stimulus alone trials, and then 10 sets of the following four trial types counterbalanced to control for order: 40-ms, 115-dB noise burst alone (startle stimulus); startle stimulus preceded 100 ms by a 20-ms, 71-dB, or 77-dB noise burst; and no stimulus. Intertrial interval was variable (average 15 sec). At the end of this block of 40 trials, the animal again received four startle stimulus alone trials followed by four no-stimulus trials.
Homogeneous Set Tests for Aggressive Behaviors.
Pairs of body weight matched males (6–7 months old, individually housed) from the same genotype were tested in a clean neutral cage (30 × 20 × 13 cm) over 3 consecutive days. They first were placed in either side of the test cage, which was separated by a transparent acrylic board in the center. After a 5-min adaptation period, the divider was removed, and males were tested for aggression for 15 min. For each pair, latency to the first aggressive behavioral act (except tail rattling; 900 sec was given to nonaggressive pairs) and total number of aggressive bouts were scored in a blind fashion. An aggressive bout was defined as a continuous series of behavioral interactions including at least one aggressive behavioral act (see below). Three seconds was the maximum amount of time that could elapse between aggressive behavioral acts to be considered part of the same aggressive bout: if intervals between the occurrences of two behavioral aggressive acts exceeded 3 sec, the two behavioral acts were scored as two separate aggressive bouts. Aggressive behavior acts consisted of tail rattling, chasing, boxing, biting, offensive attack (often accompanied by biting), and wrestling.
RESULTS
Generation of COMT-Deficient Mice.
To generate COMT-deficient mice, a human comt cDNA probe was used to screen a mouse brain cDNA library. A full-length cDNA clone was isolated (Fig. 1A), and part of it was used to screen a mouse 129/Sv genomic library. A positive phage encompassing the entire set of comt coding exons was isolated and used to prepare a targeting construct (Fig. 1B). Heterozygous mice showed the expected pattern of gene disruption by Southern (Fig. 1C) and PCR analysis. They were viable and fertile and were intercrossed to obtain homozygous COMT-deficient mice. Homozygous mice were obtained at the expected frequencies, and they were apparently physically healthy and fertile and gained weight normally. Brain morphology appeared identical in both null mice and their wild-type littermates by gross evaluation. Upon microscopic examination of sections, cell groups in the forebrain and diencephalon appeared to be well formed with no obvious neuroanatomical alterations. In addition, immunocytochemistry using a tyrosine hydroxylase antibody failed to reveal any major anomalies in the distribution of dopaminergic neurons (data not shown).
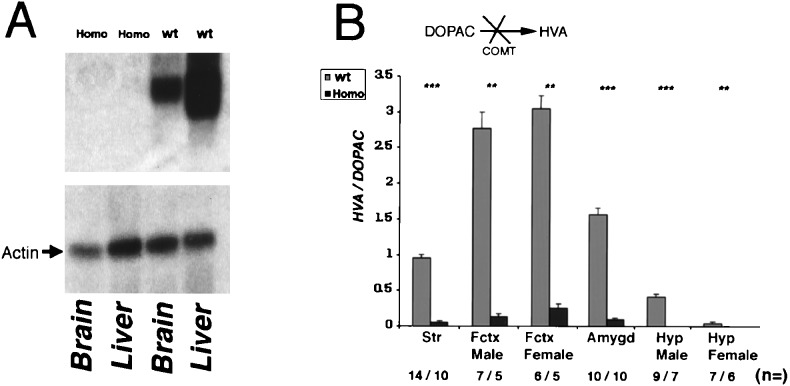
Northern analysis of total RNA isolated from liver and brain of homozygotes detected no COMT-encoding mRNA (Fig. 2A). In addition to dopamine, COMT methylates dopamine’s hydroxylated metabolite DOPAC, resulting in the formation of HVA (1). Therefore the ratio of HVA/DOPAC can be used as a reliable measure of COMT activity, independent of initial dopamine concentration. Comparisons in homozygous and wild-type animals using the nonparametric Mann–Whitney u test showed significant decreases in the HVA/DOPAC ratio in all brain regions tested (Fig. 2B).
Figure 2.
(A) Northern blot analysis of mRNA isolated from the liver and the brain of homozygous and wild-type animals. Two mRNA species are observed in the liver, corresponding (by analogy to the rat and human gene) to two distinct sites of transcriptional initiation. The coding part of the COMT cDNA was used as probe. As a control the Northern blot was probed with a probe from rat β actin. (B) HVA/DOPAC ratio in the striatum, frontal cortex, amygdala, and hypothalamus of male and female homozygous and wild-type animals. Values are the average ± SEM for wild-type (shaded bar) and homozygous (solid bar) mice. Differences between wild-type and homozygous mice tested by Mann–Whitney u test were ∗∗∗, P < 0.0002, ∗∗, P < 0.002. The calculated HVA/DOPAC ratios for homozygous (Homo) versus wild-type (wt) animals are as follows: HVA/DOPACstriatum = 0.05 ± 0.009 vs. 0.94 ± 0.71 (P = 0.0001); HVA/DOPACfrontal cortex/males = 0.13 ± 0.04 vs. 2.77 ± 0.59 (P = 0.003); HVA/DOPACfrontal cortex/females = 0.25 ± 0.09 vs. 3.05 ± 0.37 (P = 0.002); HVA/DOPACamygdala = 0.09 ± 0.009 vs. 1.56 ± 0.17 (P = 0.0002); HVA/DOPAChypothalamus/males = 0 vs. 0.41 ± 0.05 (P = 0.0006); and HVA/DOPAChypothalamus/females = 0 vs. 0.04 ± 0.008 (P = 0.002); It is notable that although the HVA/DOPAC ratio is decreased significantly in all of the brain areas tested, residual HVA is detectable, in several of these areas (not shown). One interpretation is that the residual methylation of dopamine and DOPAC is caused by the action of yet unidentified methyltransferases involved in the clearance of dopamine, whose activity is probably up-regulated in the absence of COMT. Further work is needed to verify this interpretation and possibly lead to better characterization of this pathway. Str, striatum; Fctx, frontal cortex; amygd, amygdala; Hyp, hypothalamus. The number of the animals tested (n =) is indicated.
Sexually Dimorphic Neurochemical Changes in COMT-Deficient Mice.
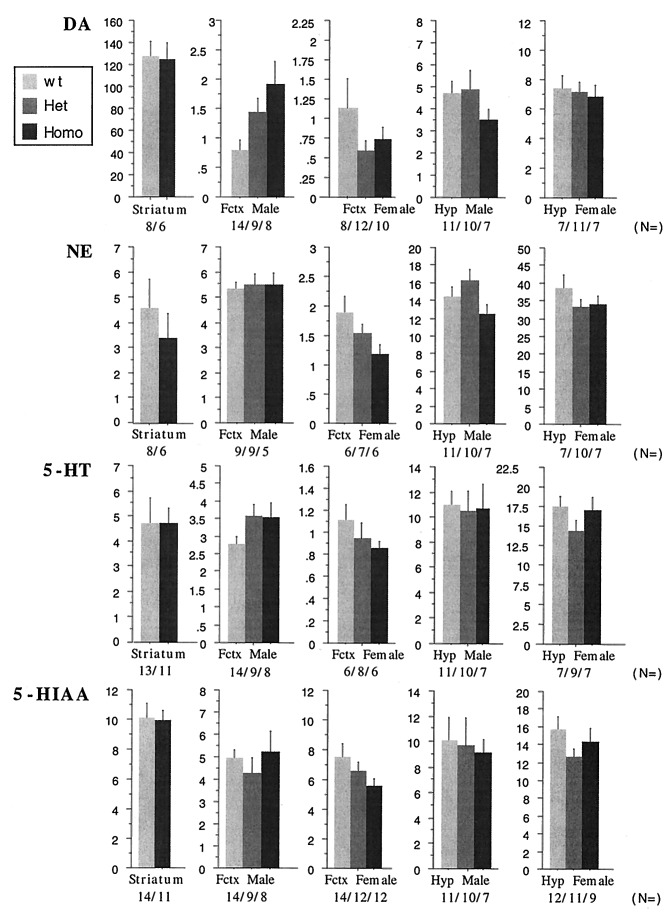
As a first step toward understanding the consequences of the absence of COMT on neurotransmission in several brain regions, steady-state levels of dopamine and norepinephrine were compared in the striatum, frontal cortex, and hypothalamus of homozygous and wild-type animals. Based on our previous observations, which suggested a sexually dimorphic contribution of COMT in psychiatric phenotypes (10), as well as on earlier observations by others (12), male and female animals were analyzed separately (Fig. 3). Unlike the MAO-A and MAO-B knockout mice, where no changes in the dopamine levels were observed (18, 19), a 2- to 3-fold increase in the amount of dopamine in the frontal cortex of male homozygous mice was noted (1.916 ± 0.375 vs. 0.785 ± 0.163), demonstrating the importance of COMT for dopamine metabolism. Interestingly, female homozygous animals did not demonstrate a similar increase (presumably because of compensation). In contrast, and despite striking changes in the HVA/DOPAC ratio, no accompanying changes of dopamine content were recorded in either hypothalamus or striatum of either males or females. This latter observation is in agreement with previous microdialysis studies in rats where administration of COMT inhibitors—despite pronounced changes in dopamine metabolites—failed to affect the levels of striatal dopamine, unless dopamine carrier system or MAO-A activity were inhibited by nomifensine or clorgyline, respectively (20). The increase of the dopamine levels in the male frontal cortex as compared with striatum is also in agreement with previous observations that O-methylation is a prominent step in the clearance of dopamine in the frontal cortex but not in the striatum (2). Norepinephrine, 5-hydroxytryptamine, and 5-hydroxy indole acetic acid levels did not change significantly in any region tested in our sample (Fig. 3).
Figure 3.
Effect of COMT gene disruption on dopamine (DA), norepinephrine (NE), 5-hydroxytryptamine (5-HT), and 5-hydroxy indole acetic acid (5-HIAA). Shown are steady-state levels (in pg/μg protein) in the striatum, frontal cortex (Fctx), and hypothalamus (Hyp) of homozygous, heterozygous, and wild-type animals of both sexes (with the exception of striatum where heterozygous animals were not analyzed). Data were analyzed by two-way ANOVA. Dopamine levels for frontal cortex as shown by ANOVA were F(2,28) = 6.005, P < 0.01; norepinephrine levels for hypothalamus were P = 0.051 (a comparison of heterozygous to wild-type and homozygous males was short of significant). In the case of the striatum, data from males and females were pooled and differences between wild-type and homozygous animals were tested by Student’s t test. For all other areas, male and female data are presented even though no sex differences were present. The number of the animals tested (n = ) is indicated.
Behavioral Phenotype of COMT-Deficient Mice.
Dopamine is involved in controls over motor function and affect, as well as central processes (such as sensorimotor gating) affected in patients with psychiatric disorders (21, 22). Given our initial neurochemical observations in the brain of COMT-deficient mice, a number of relevant behaviors were investigated in both sexes separately.
Spontaneous locomotor activity was tested in an open-field apparatus (23) equipped with infrared sensors for the automatic recording of horizontal activity. To minimize the influence of anxiety on activity level, animals were handled and pre-exposed to the chamber before testing, and activity was monitored under indirect, very dim light and sound-attenuated conditions (unlike the quite aversive classical open-field assays performed under bright light). No significant differences in activity or in stereotypic behavior were observed among homozygous and wild-type animals of either sex (data not shown).
Having established that no locomotor deficits are present in the COMT-deficient mice, the effect of the COMT mutation on anxiety-like behaviors (collectively termed anxiety, reactivity, or emotionality) was recorded in a dark/light exploratory model in a two-compartment dark/light box, a modified open-field apparatus, where an enclosed black acrylic box was inserted into the right half of the activity chamber with an opening allowing for passage between a dark and a brightly lit open compartment. Previous work assessing the effects of anxiolytic and anxiogenic agents has established the validity of this procedure in evaluating anxiety-like behaviors in rodents (24–26). Variables recorded as a measure of anxiety included latency to emerge from the dark compartment into the more aversive brightly lit compartment and amount of time spent ambulating in each of the two compartments.
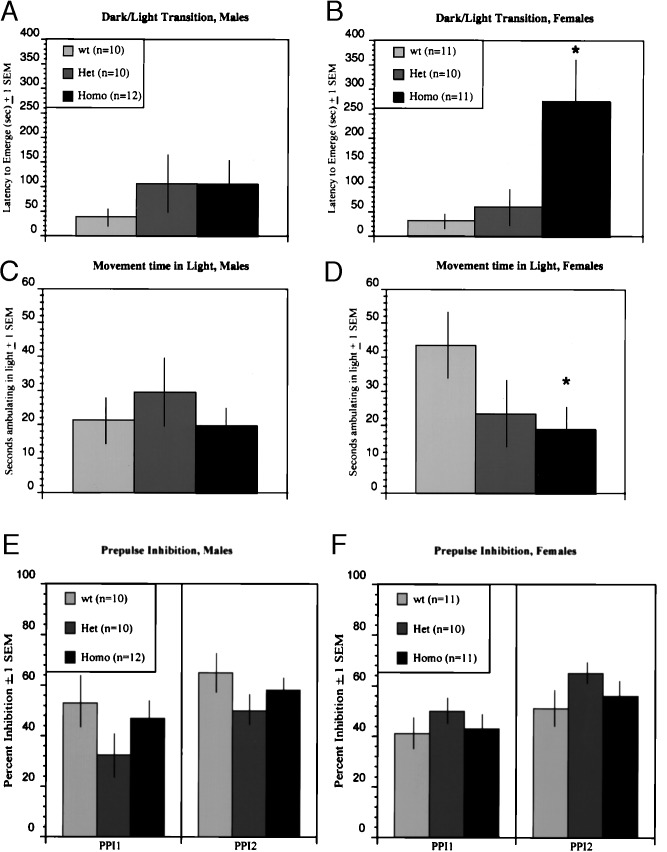
An ANOVA of genotype by latency to emerge was significant for females (P < 0.01). Specifically, homozygous COMT-deficient females demonstrated increased anxiety, as they were more reluctant to emerge into the light (it took them on average about nine times longer to emerge), and in addition, once they emerged, they were significantly less ambulatory than wild-type animals (Fig. 4 B and D). Comparison of wild-type and homozygous females on time spent ambulating in the dark was not significant, consistent with our analysis in the very dimly lit open-field test, suggesting that the observed differences in the dark/light exploratory model are specific and cannot be attributed to generalized locomotor dysfunction of the COMT-deficient mice. In direct contrast, no significant effect of genotype on latency to emerge or time spent ambulating in the light was observed for COMT-deficient males (see Fig. 4 A and C).
Figure 4.
Effect of COMT gene disruption on emotionality and sensory gating. Homozygous females took longer to emerge into the light than did wild-type females (B), and they also spent less time ambulating in the light compartment (D). An ANOVA of genetype by latency to emerge was significant (2,29) = 6.056; P < 0.01 for females. Follow-up multiple comparison tests using the Bonferroni correction showed that female homozygous differed from both wild-type (P < 0.05) and heterozygous (P < 0.05) female mice, whereas the latter two groups did not differ from each other. A planned comparison of wild-type and homozygous females on total time spent in the lgiht was short of significant (not shown). However, planned comparison of wild-type and homozygous females on time spent ambulating in the light was significant [(20) = 2.12; P < 0.05], whereas the same comparison for ambulation in the dark was not (P = 0.56). Males did not differ on either measure of emotionality (A and C). Prepulse inhibition was examined for two prepulse dB levels; higher y-axos values represent greater percent inhibition. There were no effects of genetype for either males (E) or females (F) (∗, P < 0.05).
Sensorimotor gating is a central processing mechanism that is affected in patients with schizophrenia and OCD (16, 22, 27) (neuropsychiatric disorders associated with hemizygous 22q11 deletions) (6). Attenuation of the startle response by PPI provides a measure of sensorimotor gating. PPI occurs when an abrupt startling acoustic stimulus is preceded 30–500 msec by a barely detectable prestimulus or “prepulse.” Mice demonstrate a robust and reliable PPI, which can be disrupted by drugs such as apomorphine and PCP that are known to interfere with dopamine neurotransmission (17). PPI was recorded in COMT-deficient mice as described in Materials and Methods and expressed as 100 − [(response to startle stimulus after prepulse/response to startle stimulus alone) × 100], such that higher percentages represent greater levels of inhibition (Fig. 4 E and F). ANOVAs conducted separately for the two prepulse levels tested, produced no effect of genotype, for either females or males.
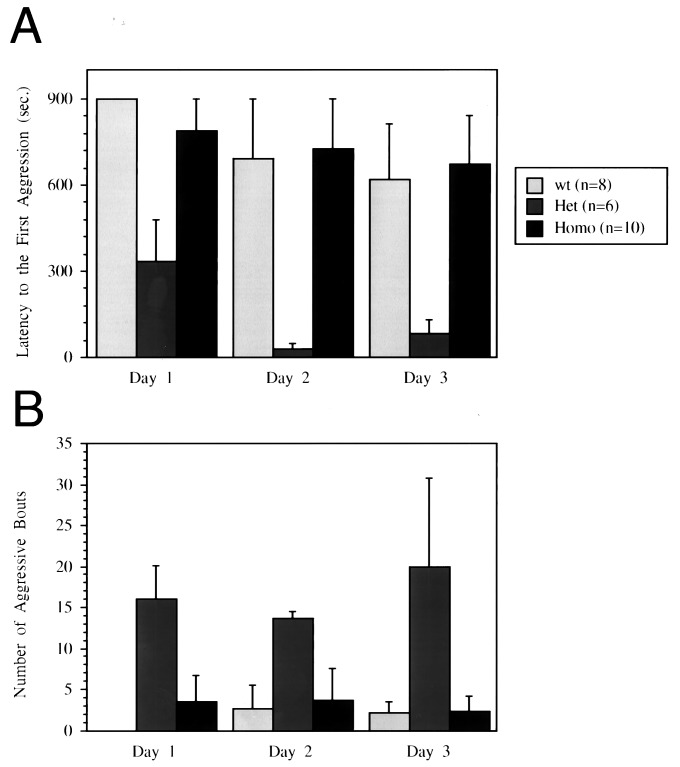
It previously was shown that male mice deficient in MAO-A manifested enhanced aggression (18). Both serotonin and norepinephrine levels (but not dopamine levels) were increased in the brains of these animals (18). Our initial attempts to house mice grouped by genotype after weaning led to consistent observations of fighting among heterozygous male mice. This observation prompted us to investigate more systematically the pattern of aggressive behavior in male mice of all three genotypes by using, as the most appropriate test in this case, the homogeneous set test. Pairs of body weight-matched males from the same genotype were tested in a clean neutral cage over 3 consecutive days. Latency to the first aggressive behavioral act (Fig. 5A; P < 0.05) as well as total number of aggressive behavior bouts during 15-min tests (Fig. 5B; P < 0.01) were significantly different between genotypes. As expected, and in contrast to the other behavioral traits described above, aggressive behavior was greatly increased in heterozygous mice compared with the other two genotypes. Heterozygous pairs showed significantly higher frequency of aggressive behavior with shorter latencies compared with the other two genotypes. It should be noted that increased aggressiveness of the heterozygous male mice also was observed in a pilot resident-intruder aggression test. In this test, mice of all three genotypes were tested in their home cage against olfactory bulbectomized Swiss–Webster male intruder mice, which were not aggressive at all (two of two pairs including heterozygous mice and none of the four pairs including wild-type and homozygous mice demonstrated increased aggression, data not shown).
Figure 5.
Effects of COMT gene disruption on aggressive behaviors. Data were analyzed by ANOVA for repeated measurements for the main effects of genotype and test day and their interaction. (A) Mean (+ SEM) latency to the first aggressive behavior act. There were overall genotype differences [F(2,9) = 5.057, P < 0.05], and heterozygotes showed significantly shorter latency to aggression (P < 0.05) compared with both wild-type and homozygotes, which were not different from each other. It also was found that latency decreased over 3 days [F(2,18)= 4.838, P < 0.05]. (B) Significant genotype differences were found in the total number of aggressive behavior bouts during 15-min tests [F(2,9) = 8.708, P < 0.01]. Heterozygous pairs were significantly more aggressive (P < 0.05) than both wild-type and homozygous pairs. The latter two groups were not different from each other, although further testing with an additional set of animals (that were not included in the present analysis because of differences in previous experiences) indicated a trend in which wild-type mice became more aggressive than homozygous mice after repeated behavioral tests for aggression (data not shown).
DISCUSSION
Sexually Dimorphic Effects on Steady-State Catecholamine Levels and Behavior.
Our results provide evidence for an important sex- and region-specific contribution of COMT in the maintenance of steady-state levels of catecholamines in the brain and suggest a role for COMT in some aspects of emotional and social behavior in mice. In general, the neurochemical changes observed in the COMT-deficient mice can be attributed to sexually dimorphic and region-specific patterns of (i) dopamine/norepinephrine metabolism, neurotransmission, or densities of dopamine/norepinephrine neurons/terminals and (ii) compensatory changes in dopamine/norepinephrine metabolism, neurotransmission or densities of dopamine/norepinephrine neurons/terminals, induced by the chronic absence of COMT activity. Differences in compensation efficiencies between male and female mice (directed by differences in the hormonal environment) may underlie, for example, the observed sexual dimorphism in the cortical dopamine levels of homozygous mice. Interestingly, null mutants also present sex-specific behavioral deficits that might be related to the observed (or yet to be identified) sexually dimorphic alterations in catecholamine content.
Specifically, COMT-deficient female mice demonstrated altered emotional reactivity in the dark/light exploratory model, providing evidence for a role of COMT in the control of some aspects of emotionality in mice. In sharp contrast, sensory reception and processing were unaffected, at least for the stimuli and for the genetic background tested. Behavioral and pharmacological studies on inbred lines using the dark/light exploratory model of anxiety suggest that the type of emotional behavior assayed in the present study is a central nervous system state with a genetic basis (25, 28). Moreover, based on the differential effect of certain drugs on the light/dark choice versus the open-field exploratory model, it was proposed that the novelty-induced behavior in the open-field model is related to “trait” anxiety, whereas the behavior of mice in the light/dark choice model is related to “state” anxiety induced by fear-provoking situations (26, 29). However, in the absence of any information on dynamic neurochemical changes and because of the widespread distribution and the pleiotropic effects of catecholamines, an exact correlation between region-specific neurochemical changes and specific behaviors is still unclear and is the subject of further genetic/pharmacological manipulations.
Gene Dosage Effect on Behavior in COMT-Deficient Mice.
In the present study, COMT-deficient heterozygous male mice exhibited increased aggressive behavior. It should be noted that increased aggression has been reported for psychiatric patients homozygous for the comt low activity allele (30). Aggression is a relatively complex stereotypic behavior that is affected directly or indirectly by a number of genes. Although the role of COMT in aggression remains to be determined, the most important implication of our observation is that a 2-fold decrease in COMT activity can produce a behavioral phenotype. Human subjects homozygous for the low activity comt allele present 25–35% of the maximum COMT activity (31), an activity level comparable to the expected level in heterozygous COMT mutant mice. We previously have shown that male subjects carrying the low activity genotype are at risk to develop OCD (10). The same is true for patients carrying only one copy of the comt gene because of a hemizygous 22q11 deletion.
Interestingly, increased aggression was not observed among homozygous COMT null mutant mice. Although, at first surprising, a similar pattern of genotype/aggression interaction has been described previously for male mice deficient for another relatively widely distributed protein, the α-calcium-calmodulin kinase II (32). One explanation of this nonmonotonic gene dosage effect is that in heterozygous males, half gene dosage brings COMT activity above the threshold for induction of compensatory changes with phenotypic consequences on behavior. In contrast, in homozygous males activity reduction below this threshold induces compensatory changes. Another explanation assumes that aggressive behavior is positively or negatively controlled by a number of brain regions whose function may depend or not on COMT activity. Brain regions that exert a negative effect on aggressive behavior in a COMT-dependent manner may have intrinsically low levels of COMT activity and thus are affected by half dosage in heterozygous male mice, resulting in increased aggression. In contrast, COMT-dependent regions that affect aggression positively may have intrinsically higher levels of COMT activity and thus are affected only in homozygous mice, compensating for the “heterozygous effect.” High-resolution neurochemical analysis in the brains of COMT-deficient mice of both genotypes will be necessary to test the validity of this model. Other models also could theoretically be applied that have to take into consideration the interplay between a simple genetic lesion and the complex organization of brain and behavior.
COMT-Deficient Mice as a Model for Psychiatric Disorders.
A mouse knockout model for a gene considered a candidate for a common, complex psychiatric disorder is unlikely to serve as model for the entire complexity of the disorder for at least two reasons. First, individual disease susceptibility alleles are neither necessary nor sufficient to cause the disorder, but rather interact with a number of other disease alleles to confer increased risk. Second, when compared with the complexities of human behaviors, mice present a relatively simpler spectrum of stereotypic behaviors. Although neurotransmitter systems (such as the catecholamine system) are likely to affect many of these behaviors in humans and mice, whether the affected behaviors are “homologous” is a matter of debate (33, 34). Accordingly, considerable caution has to be taken in extrapolating results obtained in mice to explain abnormal human behavior. Nevertheless, a mouse knockout model for a gene considered a candidate for a psychiatric disorder can provide a framework for understanding the involvement of this gene by providing clues regarding its mode of function. In the case of COMT, the region-specific and sexually dimorphic alterations in the content of dopamine observed in the COMT-deficient mice and the implied sex differences in compensatory mechanisms may serve as a model to understand sex-specific penetrances of mutations predisposing to psychiatric disorders. They also may have parallels in human subjects with low COMT activity and could underlie a contribution of low COMT activity in susceptibility to psychiatric disorders characterized by sex differences in clinical manifestations (10). In addition, the increase of dopamine levels in the frontal cortex of male mice may provide insights into the association between low COMT activity and OCD in males. Several studies have implicated frontal cortex dysfunction in the pathogenesis of OCD (35). Moreover, augmentation with dopamine receptor blockers has proven useful among the ≈40% of OCD patients who do not respond to the selective serotonin reuptake inhibiting agents (36, 37), thus implicating hyperactivity of dopaminergic pathways in the illness.
Acknowledgments
We thank Keesook Lee for the embryonic stem cell work and Qiarong Jiang for technical help with the animal colony.
ABBREVIATIONS
- COMT
catechol-O-methyltransferase
- MAO
monoamine oxidase
- OCD
obsessive compulsive disorder
- HVA
homovanillic acid
- DOPAC
dihydroxyphenyl acetic acid
- PPI
prepulse inhibition
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF076156).
References
- 1.Napolitano, A., Cesura, A. M. & Da Prada, M. (1995) J. Neural Transm. 45, Suppl., 35–45. [PubMed]
- 2.Karoum F, Chrapusta S J, Egan M F. J Neurochem. 1994;63:972–979. doi: 10.1046/j.1471-4159.1994.63030972.x. [DOI] [PubMed] [Google Scholar]
- 3.Boudikova B, Szumlanski C, Maidak B, Weinshilboum R M. Clin Pharmacol Ther. 1990;48:381–389. doi: 10.1038/clpt.1990.166. [DOI] [PubMed] [Google Scholar]
- 4.Cohn C K, Axelrod J. Life Sci. 1971;10:1351–1354. doi: 10.1016/0024-3205(71)90335-3. [DOI] [PubMed] [Google Scholar]
- 5.Ladosky W, Schneider H T. Br J Med Biol Res. 1981;14:409–413. [PubMed] [Google Scholar]
- 6.Karayiorgou M, Morris M A, Morrow B, Shprintzen R J, Goldberg R, Borrow J, Gos A, Nestadt G, Wolyniec P S, Lasseter V K. Proc Natl Acad Sci USA. 1995;92:7612–7616. doi: 10.1073/pnas.92.17.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan W, Jacobsen L K, Krasnewich D M, Guan X Y, Lenane M C, Paul S P, Dalwadi H N, Zhang H, Long R T, Kumra S. Am J Med Genet. 1998;81:41–43. [PubMed] [Google Scholar]
- 8.Papolos D F, Faedda G L, Veitm S, Goldberg R, Morrow B, Kucherlapati R, Shprintzen R J. Am J Psychiatry. 1996;153:1541–1547. doi: 10.1176/ajp.153.12.1541. [DOI] [PubMed] [Google Scholar]
- 9.Karayiorgou M, Gogos J A, Galke B L, Wolyniec P S, Nestadt G, Antonarakis S E, Kazazian H H, Housman D E, Pulver A E. Biol Psychiatry. 1998;43:425–431. doi: 10.1016/s0006-3223(97)00202-3. [DOI] [PubMed] [Google Scholar]
- 10.Karayiorgou M, Altemus M, Galke B L, Goldman D, Murphy D L, Ott J, Gogos J A. Proc Natl Acad Sci USA. 1997;94:4572–4575. doi: 10.1073/pnas.94.9.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gershon E S, Jonas W Z. Arch Gen Psychiatry. 1975;32:1351–1356. doi: 10.1001/archpsyc.1975.01760290019001. [DOI] [PubMed] [Google Scholar]
- 12.Puzynski S, Bidzinski A, Mrozek S, Zaluska M. Acta Psychiatry Scand. 1983;67:96–100. doi: 10.1111/j.1600-0447.1983.tb06728.x. [DOI] [PubMed] [Google Scholar]
- 13.Li T, Vallada H, Curtis D, Arranz M, Xu K, Cai G, Deng H, Liu J, Murray R, Liu X, Collier D A. Pharmacogenetics. 1997;7:349–353. doi: 10.1097/00008571-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Franklin K B J, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. New York: Academic; 1997. [Google Scholar]
- 15.Renner K J, Luine V N. Life Sci. 1984;34:2193–2199. doi: 10.1016/0024-3205(84)90320-5. [DOI] [PubMed] [Google Scholar]
- 16.Swerdlow N R, Braff D L, Taaid N, Geyer M A. Arch Gen Psychiatry. 1994;51:139–154. doi: 10.1001/archpsyc.1994.03950020063007. [DOI] [PubMed] [Google Scholar]
- 17.Paylor R, Crawley J N. Psychopharmacology. 1997;132:169–180. doi: 10.1007/s002130050333. [DOI] [PubMed] [Google Scholar]
- 18.Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, Muller V, Agnet M, Babinet C, Shih J C. Science. 1995;268:1763–1766. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grimsby J, Toth M, Chen K, Kumazawa T, Klaidman L, Adams J D, Karoum F, Gal J, Shih J C. Nat Genet. 1997;17:206–210. doi: 10.1038/ng1097-206. [DOI] [PubMed] [Google Scholar]
- 20.Kaakkola S, Wurtman R J. J Neurochem. 1993;60:137–144. doi: 10.1111/j.1471-4159.1993.tb05831.x. [DOI] [PubMed] [Google Scholar]
- 21.Giros B, Jaber M, Jones S R, Wightman R M, Caron M G. Nature (London) 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 22.Perry W, Braff D L. Am J Psychiatry. 1994;151:363–367. doi: 10.1176/ajp.151.3.363. [DOI] [PubMed] [Google Scholar]
- 23.Crusio W E, Schwegler H, van Abeelen J H. Behav Brain Res. 1989;32:75–80. doi: 10.1016/s0166-4328(89)80074-9. [DOI] [PubMed] [Google Scholar]
- 24.Crawley J, Goodwin F K. Pharmacol Biochem Behav. 1980;13:167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- 25.Mathis C, Paul S M, Crawley J N. Behav Genet. 1994;24:171–180. doi: 10.1007/BF01067821. [DOI] [PubMed] [Google Scholar]
- 26.Griebel G, Sanger D J, Perrault G. Neuropharmacology. 1996;35:1081–1091. doi: 10.1016/s0028-3908(96)00080-9. [DOI] [PubMed] [Google Scholar]
- 27.Swerdlow N R, Benbow C H, Zisook S, Geyer M A, Braff D L. Biol Psychiatry. 1993;33:298–301. doi: 10.1016/0006-3223(93)90300-3. [DOI] [PubMed] [Google Scholar]
- 28.Mathis C, Neumann P E, Gershenfeld H, Paul S M, Crawley J N. Behav Genet. 1995;25:557–568. doi: 10.1007/BF02327579. [DOI] [PubMed] [Google Scholar]
- 29.Belzung C, Pineau N, Beuzen A, Misslin R. Pharmacol Biochem Behav. 1994;49:433–436. doi: 10.1016/0091-3057(94)90445-6. [DOI] [PubMed] [Google Scholar]
- 30.Strous R D, Bark N, Parsia S S, Volavka J, Lachman H M. Psychiatry Res. 1997;69:71–77. doi: 10.1016/s0165-1781(96)03111-3. [DOI] [PubMed] [Google Scholar]
- 31.Lachman H M, Papolos D F, Saito T, Yu Y-M, Szumlanski C L, Weinshilboum R M. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Chen C, Rainnie D G, Greene R G, Tonegawa S. Science. 1994;266:291–294. doi: 10.1126/science.7939668. [DOI] [PubMed] [Google Scholar]
- 33.Collier D A, Sham P C. Mol Psychiatry. 1997;2:181–183. doi: 10.1038/sj.mp.4000274. [DOI] [PubMed] [Google Scholar]
- 34.Flint J, Corley R. J Mol Med. 1996;74:515–521. doi: 10.1007/BF00204977. [DOI] [PubMed] [Google Scholar]
- 35.Nordahl T E, Benkelfat C, Semple W E, Gross M, King A C, Cohen R M. Neuropsychopharmacology. 1989;2:23–28. doi: 10.1016/0893-133x(89)90003-1. [DOI] [PubMed] [Google Scholar]
- 36.Greist J H, Jefferson J W, Kobak K A, Katzelnick D J, Serlin R C. Arch Gen Psychiatry. 1995;52:53–60. doi: 10.1001/archpsyc.1995.03950130053006. [DOI] [PubMed] [Google Scholar]
- 37.McDougle C J, Goodman W K, Leckman J F, Lee N C, Heninger G R, Price L H. Arch Gen Psychiatry. 1994;51:302–308. doi: 10.1001/archpsyc.1994.03950040046006. [DOI] [PubMed] [Google Scholar]
- 38.Grossman M H, Littrell J B, Weinstein R, Szumlanski C, Weinshilboum R M. Trans Neurosci Soc. 1992;18:70. doi: 10.1016/0024-3205(92)90386-4. (Abstr.). [DOI] [PubMed] [Google Scholar]
- 39.Zhuang Y, Soriano P, Weintraub H. Cell. 1994;79:875–884. doi: 10.1016/0092-8674(94)90076-0. [DOI] [PubMed] [Google Scholar]