Abstract
Background:
There is a documented association between affective disorders (e.g., depression and anxiety) and cardiovascular disease in humans. Chronic social stressors may play a mechanistic role in the development of behavioral and cardiac dysregulation. The current study investigated behavioral, cardiac, and autonomic responses to a chronic social stressor in prairie voles, a rodent species that displays social behaviors similar to humans.
Methods:
Female prairie voles were exposed to 4 weeks of social isolation (n=8) or pairing (control conditions; n=7). Electrocardiographic parameters were recorded continuously during isolation, and behavioral tests were conducted during and following this period.
Results:
Isolation induced a significant increase in resting heart rate, reduction in heart rate variability (standard deviation of normal-to-normal intervals and amplitude of respiratory sinus arrhythmia), and exaggerated cardiac responses during an acute resident-intruder paradigm. Isolation led also to both depression- and anxiety-like behaviors in validated operational tests. These changes in response to social isolation showed predictable inter-relations, and were mediated by a disruption of autonomic balance including both sympathetic and parasympathetic (vagal) mechanisms.
Conclusions:
These findings indicate that social isolation induces behavioral, cardiac and autonomic alterations, related to those seen after other stressors, and which are relevant to cardiovascular disease and affective disorders. This model may provide insight into the mechanisms that underlie these co-occurring conditions.
Keywords: Anxiety, Cardiovascular disease, Depression, Heart rate, Heart rate variability, Parasympathetic nervous system, Prairie voles, Sympathetic nervous system
Introduction
Affective disorders are risk factors for morbidity and mortality related to heart disease (1-6), and cardiovascular disease may induce depression or anxiety (7-9). The mechanisms that underlie these associations are not well understood. Environmental and social stressors, and the absence of positive social interactions in social species, are associated with increased vulnerability to mood and cardiovascular disorders (10-21). For instance, perceived loneliness in humans is associated with symptoms of depression and increased cardiovascular reactivity to a mental stressor (22).
Behavioral and physiological reactivity to stressors may play a role in mediating affective disorders and cardiovascular pathophysiology (22-26). Basal and stressor-induced heart rate (HR) and HR variability changes have been described in humans with affective disorders and animal models of depression (13,27-31), and also are common in heart disease (32-35). The autonomic nervous system may mediate emotional responsiveness to environmental and social stressors, potentially leading to the development of affective disorders and heart disease (17,18,36,37).
The study of behavior and cardiac function using animal models will provide insight into mechanisms underlying the association of mood and cardiovascular disorders. Previously we demonstrated that exposure to chronic stressors in rats induces behavioral and cardiovascular disturbances, mediated in part by increased sympathetic drive to the heart (13,31,38). The socially monogamous prairie vole (Microtus ochrogaster) is a rodent species that displays social behaviors similar to humans, providing a useful model for investigating mechanisms through which social factors influence physiology and behavior (39). In this species, social isolation induced anhedonia and increased neuroendocrine responsiveness to an acute resident-intruder paradigm (15). Our laboratory also described for the first time autonomic regulation of the heart in prairie voles, including high parasympathetic (vagal) cardiac tone, similar to humans (40). The high levels of social behaviors (relative to small mammals), coupled with a parasympathetic nervous system influence regulating resting cardiac function, suggest that prairie voles can provide a translational model for studying the integration of social behavior and cardiac function.
Given the interactions among the social environment, behavior, and cardiac function, we hypothesized that social isolation in prairie voles would induce basal and stressor-induced cardiac disturbances and behaviors relevant to affective disorders, and that these changes would be influenced by autonomic mechanisms. Prairie voles were exposed to 4 weeks of social isolation versus social pairing with a same-sex sibling; this isolation period was determined from previous studies demonstrating that 4 weeks of stressor exposure induced changes relevant to depression and cardiovascular pathophysiology in rodents (13,15,31). Basal cardiac function (HR, HR variability) was assessed via electrocardiographic (ECG) measurements, and stressor-induced cardiac changes were investigated during an acute resident-intruder paradigm. Affective behaviors were assessed via sucrose intake and elevated plus maze behaviors, described as valid indices of depression and anxiety, respectively (41-45). Pharmacological autonomic blockade was conducted to test the hypothesis that autonomic mechanisms underlie the isolation-induced changes.
Methods and Materials
Animals
Fifteen adult (60-90 days) female prairie voles (35-55 grams), descendants of a wild stock caught near Champaign, Illinois, were maintained on a 14/10 h light/dark cycle (lights on at 0630 h), with a temperature of 25±1° C and relative humidity of 21±4 g/m3. Animals were allowed food (Purina rabbit chow) and water ad libitum, unless otherwise specified. Offspring were removed from breeding pairs at 21 days of age and housed in same-sex sibling pairs. All procedures were conducted according to National Institutes of Health's Guide for the Care and Use of Laboratory Animals and approved by the University of Illinois Institutional Animal Care and Use Committee.
Females were chosen for these experiments because they may be especially sensitive to the effects of social stressors (15,46). Additionally, they do not show a spontaneous puberty or estrous cycle; the ovaries remain inactive until the female has physical contact with a male, allowing for the use of reproductively intact animals without the need for controlling the estrous cycle (47). Also, affective disorders are more common in women than men (48), yet female rodents are an understudied group both in behavioral and physiological investigations relating to these disorders (see 49).
General Experimental Design
Table 1 displays a timeline of all procedures; specific experimental procedures are described in the following sections.
Table 1.
Timeline of procedures.
| Procedure | Schedule |
|---|---|
| Adaptation to 2% sucrose | Days 1-7 |
| Telemetric transmitter implantation | Days 8-10 |
| Recovery in divided cages | Days 9-15 (depending on date of transmitter implantation) |
| Recovery in home cages (with siblings) | Days 13-20 (depending on date of transmitter implantation) |
Undisturbed baseline period
|
Days 20-24 (depending on date of transmitter implantation) |
| Baseline fluid intake test; body weight measurements
|
Days 25-26 (depending on date of transmitter implantation) |
Weeks 1 and 2 isolation or pairing
|
Days 27-40 |
| Week 2 fluid intake test; body weight measurements
|
Day 41 |
Weeks 3 and 4 isolation or pairing
|
Days 41-54 |
| Week 4 fluid intake test; body weight measurements
|
Day 55 |
| Post-isolation or -pairing pharmacological and behavioral tests: (a) pharmacological autonomic blockade (atenolol, atropine, both drugs), (b) elevated plus maze, (c) resident-intruder test
|
Days 57-66 [procedures (a), (b), and (c) counterbalanced between groups, 48 hours between each procedure; drug administrations in procedure (a) counterbalanced between groups, 48 hours between each drug injection] |
Sacrifice under anesthesia
|
Day 68 |
Note: All acute procedures were conducted during the light period, 3-5 hours following light onset.
Telemetric Transmitter Implantation
Wireless radio transmitters [DataSciences International (DSI), St. Paul, MN; model TA10ETA-F20] were implanted intraperitoneally under aseptic conditions, during the light period, for long-term ECG recordings. Animals were anesthetized with ketamine and xylazine (67 mg/kg and 13.33 mg/kg, respectively, sc; NLS Animal Health, Owings Mills, MD). Transmitter implantation was similar to procedures described elsewhere (50,51). Animals were housed for 5 days in custom-designed divided cages (40) to permit adequate healing of suture wounds, and then were returned to the home cages (with the sibling) to recover for an additional 5-7 days.
Radiotelemetric Recordings
ECG signals were recorded with a radiotelemetry receiver (DSI, St. Paul, MN; sampling rate 5 kHz, 12-bit precision digitizing). Activity level was monitored via the receiver (sampling rate 256 Hz). Parameters were recorded continuously during an undisturbed baseline period (3-5 days) and throughout all of the following procedures.
Social Isolation
Following surgical recovery and collection of baseline measurements, animals were randomly divided into paired (control; n=7) or isolated (n=8) conditions; only one animal from each sibling pair was studied. Isolated animals were separated from the sibling and housed individually (in a separate room, beyond smelling distance) for 4 weeks; paired animals were continually housed with the siblings during this period. Handling and cage changing were matched between the two groups.
Fluid Intake
Ad libitum 2% sucrose was available for 1 week before beginning experimental procedures to allow for adaptation to its taste. Intake of water and 2% sucrose were measured as an operational index of anhedonia, defined as reduced sucrose intake and preference relative to control animals and baseline values (13,42,43). Food and water were removed from the cage for 16 hours prior to the test; cardiac and activity parameters were monitored to ensure that the deprivation produced no adverse effects. One hour prior to the test, all animals (paired and isolated) were moved into clean, individual cages to ensure accurate fluid intake measurements of paired animals. Water and 2% sucrose were placed on the cage in premeasured bottles, and fluid intake was monitored for 1 hour. Animals were returned to the home cages immediately following the test. Fluid intake tests were conducted prior to isolation or pairing (baseline) and following 2 and 4 weeks of this period.
Elevated Plus Maze
Anxiogenic behaviors were measured in the elevated plus maze using procedures described elsewhere (52). The maze (57 cm height) consisted of two open arms of clear Plexiglas opposite to each other (49.5×10 cm), two closed arms of black Plexiglas with an open roof (49.5×10×30.5 cm), and a center section of clear Plexiglas (10×10 cm). Telemetry receivers were placed under the center and end of each arm, approximately 6 inches below the maze. The animal was placed in the center section and allowed to explore for 5 minutes; behavior was video recorded. Animals were returned to the home cage immediately following the test. Two trained, experimentally blind observers scored the duration of time and the number of entries into the open, closed, and center sections of the maze; animals were coded as entering a section of the maze when all 4 paws were in the respective section.
Resident-Intruder Test
A resident-intruder test (15,53) was conducted in a subset of paired (n=5) and isolated (n=5) animals. The paired or isolated animal (intruder) was placed into the cage of an unrelated and unfamiliar animal of the same sex (resident) for 5 minutes. Aggressive behaviors [aggressive grooming or posture, swatting, biting, thrusting, pulling, and/or attack behavior (54)] were video recorded and scored by two trained, experimentally-blind observers. ECG parameters were recorded continuously during and following the test, and the time point (after test completion) at which HR of each animal returned to within 1% of its pre-test HR value was recorded.
Selective Pharmacological Autonomic Blockade
HR was measured under the following pharmacological conditions: (a) β-adrenergic receptor blockade (sympathetic blockade; atenolol, 8 mg/kg ip; Sigma-Aldrich, St. Louis, MO), (b) cholinergic receptor blockade [parasympathetic blockade; atropine methyl nitrate (atropine), 4 mg/kg ip; Sigma-Aldrich, St. Louis, MO], and (c) dual blockade (both drugs). These doses were chosen for their ability to completely block the respective autonomic inputs to the heart according to previously published results from voles (40,55) and preliminary analyses conducted by our laboratory. Drugs were administered in a counterbalanced fashion over a 6-day period (48 hours between each drug).
Quantification of Radiotelemetric Recordings
Quantification of Telemetric Variables
Quantification of telemetric variables was conducted according to procedures described previously (40). Multiple segments of 1-5 minutes of stable, continuous data were used to evaluate HR, HR variability and activity. Data segments were matched across subjects and time points, and were used to calculate all cardiac and activity variables.
Resting Cardiac Parameters
Activity level is high in prairie voles and occurs in short bouts throughout the light and dark periods (40), therefore resting cardiac parameters were derived from ECG data sampled during a period of at least 1 hour of minimal activity [5 counts/minute (cpm) or lower], and included the average of 2-5 minute intervals of continuous ECG data collected every 30 minutes (4-15 minutes of accumulated data from each animal).
HR was evaluated using vendor software (DSI, St. Paul, MN), and R-wave detections were verified with custom-designed software. The R-R intervals were analyzed for variations using custom-designed software, and included standard deviation of all R-R (normal-to-normal; N-N) intervals [SDNN index (56)] and respiratory sinus arrhythmia (RSA).
RSA was assessed with modification of procedures described elsewhere (40,57), which have been validated in prairie voles and other species (40,58). The amplitude of RSA represents the impact of myelinated vagal efferent pathways originating in the nucleus ambiguus (see 59). The ECG signal was exported into a data file and examined to ensure all R waves were detected. Preliminary spectral analyses identified spectral peaks within the approximate frequency band in which breathing is observed in mammals of similar size (40), confirming that prairie voles express a spectral peak in the 1-4 Hz range (55,60). The R-R intervals were resampled at 20 Hz and, to comply with the assumption of stationarity, detrended with a 21-point cubic moving polynomial to remove low frequency (trend) components below 0.5 Hz. The residuals of this procedure were free of aperiodic and slow periodic processes that may have violated the assumption of stationarity. A bandpass filter was applied to define RSA by extracting only the variance in the HR spectrum between the frequencies of 1-4 Hz.
Behavioral Tests
Cardiac and activity parameters were evaluated using continuous data recorded during the elevated plus maze and resident-intruder test (multiple segments of at least 10 seconds of data that were not confounded by movement artifact). All ECG, activity and behavioral data were synchronized.
Autonomic Blockade
The peak HR response (beginning 30 minutes following each drug injection) was evaluated using continuous ECG data (40). The data were manually examined to determine the peak HR response during a window of 1 hour that included a stable ECG recording that was not confounded by movement artifact (3-10 minutes of ECG data from each animal).
Data Analysis
Care was taken not to include periods of ECG involving animal movement artifact. Data were analyzed with mixed-design analyses of variance (ANOVA) and a priori Student's t-tests. Relevant behavior-cardiac function correlations were computed using Pearson's r. A value of P<0.05 was considered statistically significant. A Bonferroni correction was used for multiple comparisons; the adjusted probability value, depending on the number of comparisons made, was used to determine whether the result was statistically significant (probability values of 0.05 are reported in the text for accuracy).
Results
Resting Cardiac Parameters
Isolation significantly increased HR and reduced HR variability relative to control conditions (Figure 1). The ANOVA for HR yielded main effects of group [F(1,13)=5.91, P<0.05], time [F(2,26)=3.66, P<0.05], and interaction [F(2,26)=17.08, P<0.05]. The two groups did not differ in baseline HR (P>0.05); following 2 and 4 weeks of isolation, HR in the isolated group was higher than its respective baseline HR [week 2: t(7)=7.05, P<0.05; week 4: t(7)=3.72, P<0.05] and that of the paired group [week 2: t(13)=3.79, P<0.05; week 4: t(13)=3.46, P<0.05]. The paired group did not differ from its respective baseline HR over time (P>0.05 for both comparisons).
Figure 1.
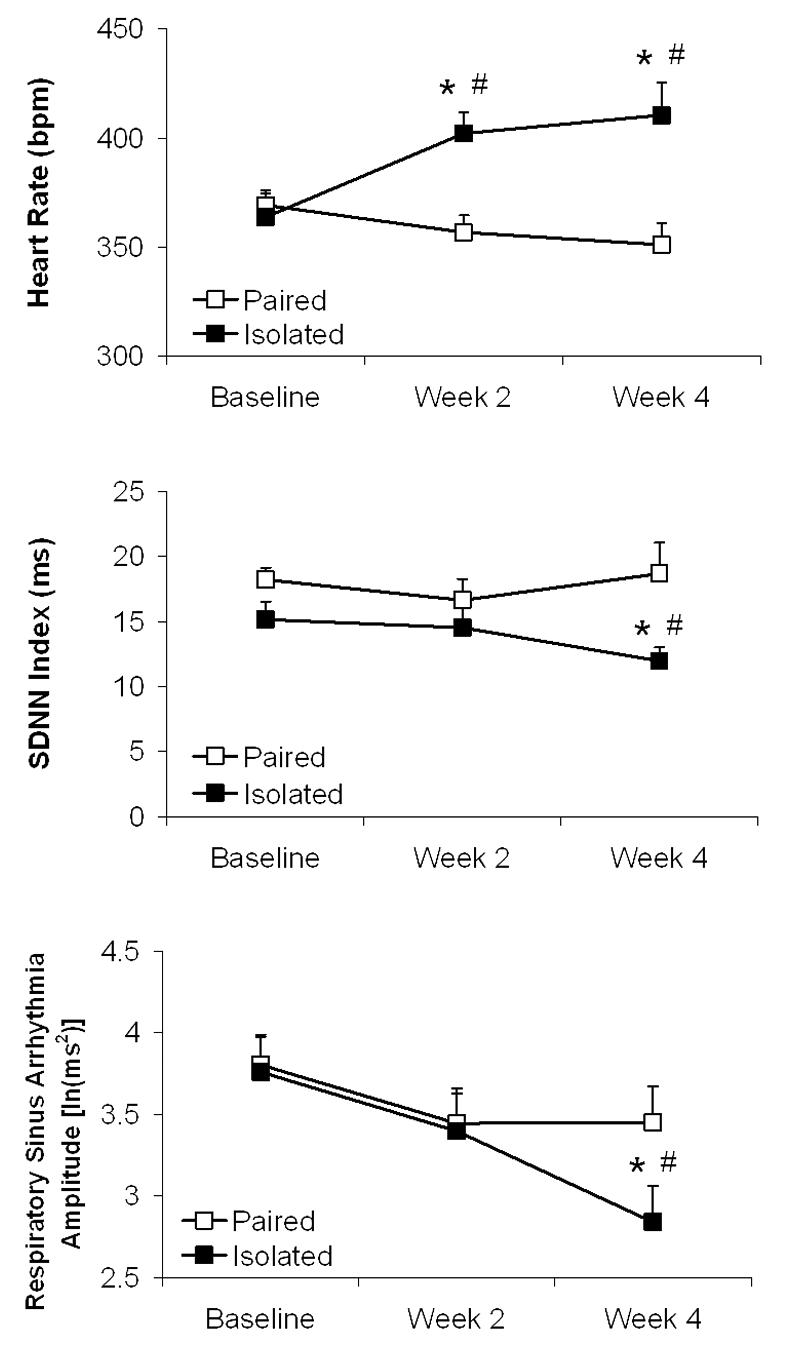
Mean (+ SEM) heart rate (Panel A), standard deviation of normal-to-normal intervals (SDNN index; Panel B), and amplitude of respiratory sinus arrhythmia (Panel C) in prairie voles at baseline and following 2 and 4 weeks of social isolation or pairing. Note the scale differences among the 3 panels. *P<0.05 vs. respective paired value; #P<0.05 vs. respective baseline value.
The ANOVA for SDNN index yielded a main effect of group [F(1,13)=6.43, P<0.05] and interaction [F(2,26)=3.38, P<0.05]. The two groups did not differ in baseline SDNN index or following week 2 (P>0.05 for both comparisons); following 4 weeks of isolation, the isolated group displayed lower SDNN index versus its respective baseline value [t(7)=2.30, P<0.05] and that of the paired group [t(13)=2.58, P<0.05]. SDNN index in the paired group did not differ over time (P>0.05 for both comparisons).
The ANOVA for RSA yielded a main effect of time [F(2,26)=7.16, P<0.05] and interaction [F(2,26)=4.05, P<0.05]. The two groups did not differ in baseline RSA amplitude or following week 2 (P>0.05 for both comparisons); following 4 weeks of isolation, the isolated group displayed lower RSA amplitude versus its respective baseline amplitude [t(7)=2,97, P<0.05] and that of the paired group [t(13)=1.95, P<0.05]. RSA amplitude in the paired group was not altered over time (P>0.05 for both comparisons).
Fluid Intake
Isolation significantly reduced sucrose intake and preference relative to control conditions, indicative of anhedonia (Figure 2). The ANOVA for water intake yielded no significant effects (P>0.05). The ANOVA for sucrose intake yielded main effects of group [F(1,13)=9.40, P<0.05], time [F(2,26)=18.41, P<0.05] and interaction [F(2,26)=39.15, P<0.05]. The two groups did not differ in baseline sucrose intake (P>0.05); following 2 and 4 weeks of isolation, sucrose consumption in the isolated group was lower than its respective baseline consumption [week 2: t(7)=3.10, P<0.05; week 4: t(7)=7.84, P<0.05] and that of the paired group [week 2: t(13)=2.13, P<0.05; week 4: t(13)=6.81, P<0.05]. The paired group did not differ from its respective baseline intake across time (P>0.05 for both comparisons).
Figure 2.
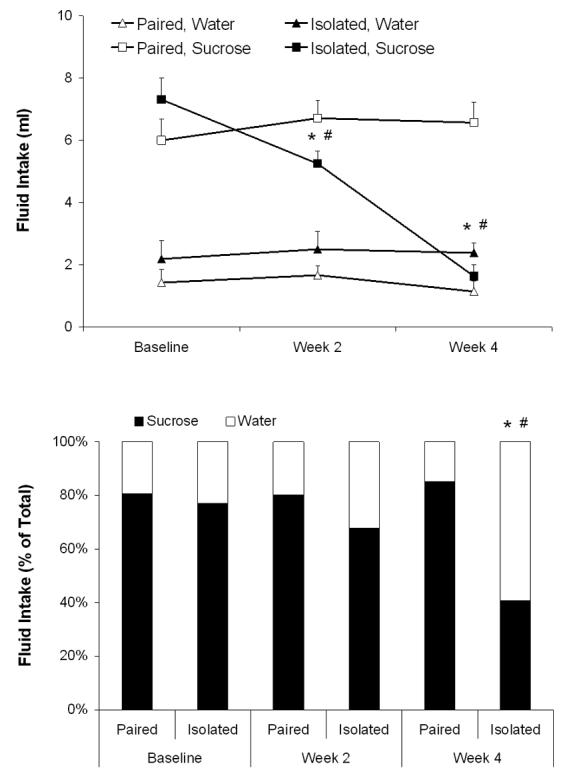
Mean (+ SEM) absolute fluid intake (Panel A) and mean sucrose preference, relative to total fluid intake (Panel B), during a 1-hour fluid intake test in prairie voles at baseline and following 2 and 4 weeks of social isolation or pairing. For sucrose intake and sucrose preference: *P<0.05 vs. respective paired value; #P<0.05 vs. respective baseline value.
The ANOVA for preference yielded main effects of group [F(1,13)=27.33, P<0.05], time [F(2,26)=12.43, P<0.05] and interaction [F(2,26)=8.92, P<0.05]. The two groups did not differ in baseline sucrose preference or following week 2 (P>0.05 for both comparisons); following 4 weeks of isolation, sucrose preference in the isolated group was lower than its respective baseline preference [t(7)=7.96, P<0.05] and that of the paired group [t(13)=5.19, P<0.05]. The paired group did not differ from its respective baseline preference across time (P>0.05).
Elevated Plus Maze
Data were excluded from one isolated animal that fell off the maze (test aborted). Isolated animals (versus paired) spent more time in the closed [t(12)=1.97, P<0.05] and center [t(12)=3.74, P<0.05], and less time in the open arms of the maze [t(12)=4.98, P<0.05], with no change in number of closed arm entries (P>0.05) or generalized locomotor activity (P>0.05), indicative of an anxiogenic response (Table 2).
Table 2.
Behavioral responses in the elevated plus maze in prairie voles following 4 weeks of social isolation or pairing.
| Duration (seconds) | Entries (total number) | Activity | |||||
|---|---|---|---|---|---|---|---|
| Open | Center | Closed | Open | Center | Closed | (cpm) | |
| Paired | 59.9 ± 8.9 | 70.1 ± 1.2 | 154.4 ± 12.4 | 2.7 ± 1.1 | 9.6 ± 1.9 | 6.4 ± 0.9 | 47.7 ± 9.7 |
| Isolated | 11.8 ± 3.8* | 103.3 ± 8.8* | 186.6 ± 10.7* | 2.3 ± 0.8 | 9.2 ± 1.8 | 6.8 ± 1.0 | 48.3 ± 2.1 |
Note: Values represent mean ± SEM.
P<0.05 vs. respective paired value.
Paired animals displayed higher HR during the open versus closed arms of the maze [t(6)=7.50, P<0.05]; HR of isolated animals did not differ between these arms (P>0.05; Figure 3). Isolated animals exhibited higher HR versus the paired group in closed [t(12) = 5.53, P < 0.05] and open [t(12) = 3.18, P < 0.05] arms of the maze.
Figure 3.
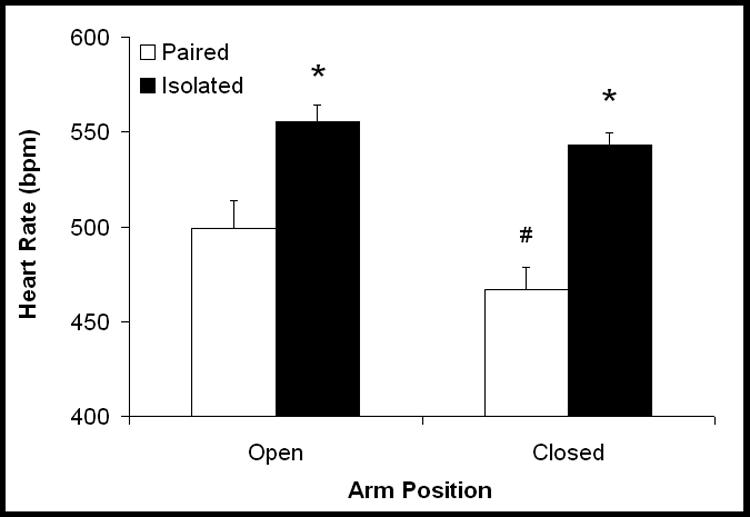
Mean (+ SEM) heart rate in prairie voles following 4 weeks of social isolation or pairing. *P<0.05 vs. respective paired value; #P<0.05 vs. respective value during the open arms.
SDNN index in the paired group was slightly (but nonsignificantly) lower during open arms versus closed arms of the maze (closed: 11.0±0.9 ms; open: 9.7±1.1 ms; P>0.05). SDNN index in the isolated group did not differ between closed and open arms (closed: 9.1±1.0 ms; open: 8.3±1.2 ms; P>0.05). No between-group differences were found (P>0.05).
No between- or within-group differences in RSA amplitude were found during the maze (P>0.05 for all comparisons; data not shown).
Resident-Intruder Test
There were no differences in aggressive behaviors (paired: 15.1±3.4 episodes; isolated: 14.4±3.8 episodes; P>0.05) or activity (paired: 36.6±6.8 cpm; isolated: 49.5±5.2 cpm; P>0.05) in paired and isolated groups during the resident-intruder paradigm.
Versus paired animals, isolated animals displayed higher HR during this paradigm [t(8)=2.21, P<0.05; Figure 4A], and more time was required for HR to recover to within 1% of pre-stressor values [t(8)=2.00, P<0.05; Figure 4B]. Versus paired animals, isolated animals displayed significantly lower SDNN index [paired: 11.5±1.1 ms; isolated: 8.4±0.7 ms; t(8)=2.32, P<0.05], but did not differ in RSA amplitude (P>0.05; data not shown).
Figure 4.
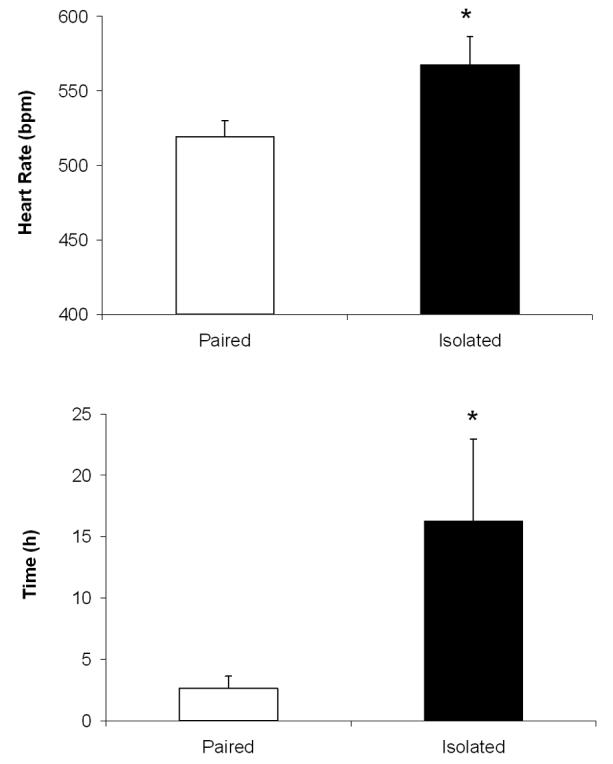
Mean (+ SEM) heart rate during a 5-minute resident-intruder test (Panel A) and time to recovery of heart rate following the test (to within 1% of pre-test heart rate values; Panel B) in prairie voles following 4 weeks of social isolation or pairing. *P<0.05 vs. respective paired value.
Selective Pharmacological Autonomic Blockade
The ANOVA for absolute HR values, relative to pre-drug HR values, yielded a main effect of drug treatment [F(3,39)=127.90, P<0.05] and interaction [F(3,39)=9.35, P<0.05; Figure 5). Absolute HR following atenolol was not different between paired and isolated groups (P>0.05); isolated animals displayed a greater reduction in HR (from pre-drug values) versus paired animals [t(13)=3.84, P<0.05]. Absolute HR following atropine [t(13)=2.85, P<0.05] and change from pre-drug values [t(13)=4.61, P<0.05] were lower in isolated versus paired animals. Absolute HR following both drugs was not different between paired and isolated groups (P>0.05); change from pre-drug values was significantly attenuated in isolated versus paired animals [t(13)=4.06, P<0.05].
Figure 5.
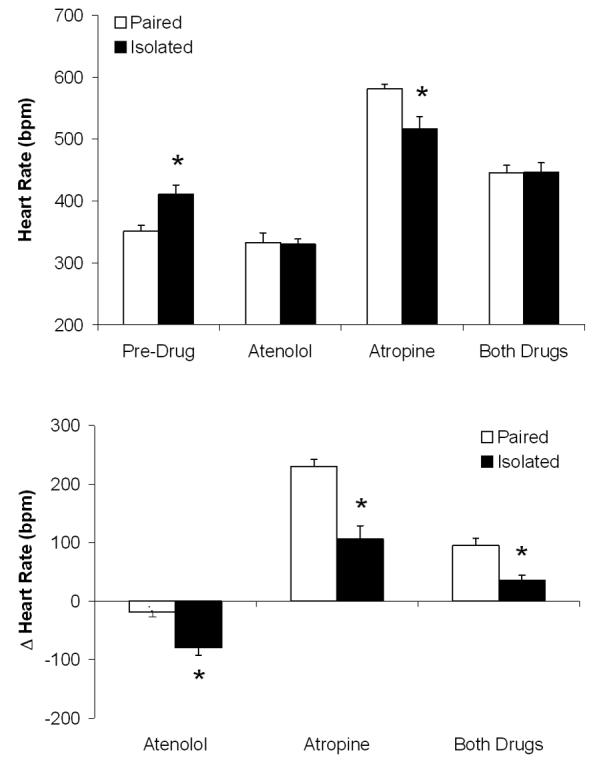
Mean (+ SEM) absolute heart rate (Panel A) relative to pre-drug values, and change in heart rate from pre-drug values (Panel B), in prairie voles following 4 weeks of social isolation or pairing and during β-adrenergic receptor blockade with atenolol, cholinergic receptor blockade with atropine, and combined receptor blockade with both drugs. *P<0.05 vs. respective paired value.
Body and Heart Weights
Table 3 displays body and heart weight, and heart:body weight ratio in paired and isolated groups. The ANOVA for body weight yielded no significant effects (P<0.05). Social isolation increased heart weight [t(13)=1.85, P<0.05] and heart:body weight ratio [t(13)=2.12, P<0.05], versus paired conditions.
Table 3.
Body weight and heart weight in prairie voles following 4 weeks of social isolation or pairing.
| Body Weight (g) | Heart Weight (g) | Heart Weight:Body Weight Ratio |
|
|---|---|---|---|
| Paired | 43 ± 3 | 0.195 ± 0.016 | 0.0045 |
| Isolated | 46 ± 3 | 0.240 ± 0.018* | 0.0053* |
Note: Values represent mean ± SEM.
P<0.05 vs. respective paired value.
Behavior-Cardiac Function Correlations
Correlations suggested several predictable inter-relations among negative affective behaviors and basal and resident-intruder-induced cardiac variables following 4 weeks of isolation (Table 4).
Table 4.
Pearson correlations among affective behaviors and basal and stressor-induced functional cardiac variables in paired and isolated prairie voles.
| Anhedonia | Anxiogenic Behavior |
|
|---|---|---|
| Heart Rate (Basal) | −0.67a* | −0.57a* |
| SDNN Index (Basal) | 0.52a* | 0.57a* |
| RSA Amplitude (Basal) | 0.42a | 0.30a |
| HR (Resident-Intruder- Induced) |
−0.43b | −0.48b |
| SDNN Index (Resident- Intruder-Induced) |
0.50b | 0.55b |
| RSA Amplitude (Resident-Intruder- Induced) |
0.22b | −0.21b |
| Anxiogenic Behavior | 0.66 a* | -- |
Note: All relations were calculated using data collected after 4 weeks of isolation or pairing. Anhedonia refers to sucrose intake; anxiogenic behavior refers to amount of time spent in the open arms of the elevated plus maze. RSA, respiratory sinus arrhythmia; SDNN, standard deviation of normal-to-normal intervals.
P<0.05
n=15
n=10.
Discussion
Social isolation induces cardiac and autonomic responses consistent with increased risk of cardiac pathophysiology, and depressive and anxiogenic behaviors. This is the first demonstration of both affective behaviors and autonomic dysfunction in prairie voles following a social stressor, extending previous findings showing that 60 days of social isolation in prairie voles induces anhedonia and increased neuroendocrine reactivity to the resident-intruder paradigm (15). This model provides insight into mechanisms through which the social environment mediates affective signs and cardiac dysfunction.
Isolation induced progressive functional cardiac changes, including elevated HR and reduced HR variability (both SDNN index and RSA amplitude). Increased HR and reduced HR variability have been described in humans with affective disorders (27-30), and an animal model of depression (13,31). These disturbances are common in heart disease, predicting mortality following myocardial infarction and heart failure (32-35). Although some functional cardiac changes were observed following 2 weeks of isolation, significant changes were not observed in all parameters until 4 weeks of isolation suggesting that a chronic underlying mechanism is likely responsible for these cardiac effects. Isolation also increased heart-to-body weight ratio. Increased ratios are found in rodent models of heart failure, and are associated with morphological changes such as left ventricular hypertrophy (9,61). The specific morphological cardiac changes in isolated prairie voles were not investigated here, but may reflect structural cardiac changes due to pathological (hypertrophy) or non-pathological (increased muscle mass) reasons.
Social isolation induced behavioral disruptions relevant to affective disorders. Anhedonia, a diagnostic feature of depression (48), was observed in isolated prairie voles. This and other depressive signs are found in rodent models of depression and acutely isolated prairie voles (13,62,63). Decreased responsiveness to sucrose is a valid and reliable operational index of depression in rodents (41). In isolated animals decreased responsiveness suggests a specific hedonic deficit similar to previous reports (13,64); reduced sucrose consumption was not due to lower body weight, and there was no indication of a deficit in generalized ingestive behavior (water intake remained unchanged).
Similar to previous results (44,45), isolated animals spent less time in open sections and more time in closed sections of the elevated plus maze, with no generalized activity changes, suggesting anxiogenic behaviors. Additionally, to our knowledge this study provides the first description of continuous cardiac function during the elevated plus maze. Paired animals exhibited increased tachycardic responses to the open versus closed arms; this response pattern provides additional validity to the behavioral measures employed in this task, suggesting that the unprotected arms are interpreted as more threatening than the protected arms. Moreoever, HR responses in isolated animals were higher than paired, and did not differ between closed and open arms, suggesting that isolated animals did not distinguish (physiologically) between protected and unprotected arms of the maze. Further studies should extend the association of behavioral and cardiovascular reactivity in this paradigm.
Increased reactivity to acute stressors is directly linked to psychological and physiological disorders (65-67). Isolation increased cardiac responsiveness to the resident-intruder stressor. Similar to previous findings (15), paired and isolated animals did not differ in generalized activity level or number of aggressive behaviors, suggesting that this paradigm represents a stressor in prairie voles. Despite these behavioral similarities, the isolated group (versus paired) exhibited increased HR and reduced HR variability, and required a longer period for HR to recover to pre-stressor values. Similar acute stressor-induced responses have been observed in a rat model of depression (13,31).
We examined a potential autonomic mechanism underlying the behavioral and cardiac changes in isolated prairie voles. Autonomic imbalance predisposes subjects to ventricular arrhythmias, including ventricular fibrillation (68-70). Attenuated increases in HR following atropine suggests that isolation reduces vagal control of the heart. Exaggerated reduction in HR following atenolol suggests that sympathetic cardiac tone may also be elevated. It is possible that higher resting HR led to the exaggerated response to atenolol in the isolated group. However, HR variability responses may provide additional insight into these autonomic changes. SDNN reflects spontaneous changes in sinus rate, representing the influence of sympathetic and parasympathetic inputs converging on the cardiac pacemaker (71); reduced SDNN may result from increased sympathetic and/or decreased parasympathetic cardiac tone, possibly involving catecholaminergic or dopaminergic mechanisms (see discussion in 6). Alternatively, RSA is a sensitive index of parasympathetic cardiac control, reflecting the influence of pathways innervating the heart via the myelinated vagus (59). Reduced SDNN and RSA suggest that both sympathetic and parasympathetic mechanisms are altered during isolation. Further, HR was significantly changed (but RSA was not) following 2 weeks of isolation, suggesting a predominant sympathetic mechanism may underlie HR changes early during isolation whereas both sympathetic and vagal mechanisms may regulate cardiac responses during longer isolation periods and behavioral tasks. Consistent with this conclusion are findings that both sympathetic and parasympathetic mechanisms mediate ventricular arrhythmias during the resident-intruder paradigm in rats (50)
The present findings are extended by the examination of relations among affective behaviors and basal and resident-intruder-induced cardiac variables after isolation. As expected, a greater degree of anhedonia (lower sucrose intake) and a greater degree of anxiogenic behavior (more time in the open arms of the elevated plus maze) both were related to higher HR and lower SDNN index following isolation and during the resident-intruder paradigm, and to lower RSA amplitude following 4 weeks of isolation. Similarly, anhedonia was positively related to anxiogenic behavior. Although the sample sizes are not large enough to yield correlations that are significantly different from zero for all correlations (see Table 4), these inter-relations suggest the importance of studying individual vulnerability to social stressors in socially monogamous species.
This study provides an integrative investigation of behavior and cardiac function in a socially monogamous rodent model system. Social isolation induces responses relevant to both affective and cardiovascular disorders. Both reduced vagal and increase sympathetic tone may underlie these detrimental effects. These findings indicate that chronic absence of positive social interactions may contribute to the development of affective disorders and cardiovascular disease, and increase our understanding of how the social environment regulates behavior and physiology in female humans.
Acknowledgements
The authors are grateful to Ms. Narmda Kumar and Mr. Eric Schmidt for technical assistance. This research was supported by National Institutes of Mental Health grants MH73233 (AJG), MH67446 (SWP), and MH72935 (CSC), and National Institute of Child Health and Human Development grant HD38490 (CSC).
Footnotes
Financial Disclosures:
Grippo, Angela J.: None
Lamb, Damon G.: None
Carter, C. Sue: None
Porges, Stephen W.: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Barefoot JC, Schroll M. Symptoms of depression, acute myocardial infarction, and total mortality in a community sample. Circulation. 1996;93:1976–1980. doi: 10.1161/01.cir.93.11.1976. [DOI] [PubMed] [Google Scholar]
- 2.Frasure-Smith N, Lespérance F, Talajic M. Depression and 18-month prognosis after myocardial infarction. Circulation. 1995;91:999–1005. doi: 10.1161/01.cir.91.4.999. [DOI] [PubMed] [Google Scholar]
- 3.Penninx BWJH, Beekman ATF, Honig A, Deeg DJH, Schoevers RA, van Eijk JTM, et al. Depression and cardiac mortality: results from a community-based longitudinal study. Arch Gen Psychiatry. 2001;58:221–227. doi: 10.1001/archpsyc.58.3.221. [DOI] [PubMed] [Google Scholar]
- 4.Kawachi I, Sparrow D, Vokonas PS, Weiss ST. Symptoms of anxiety and risk of coronary heart disease. The Normative Aging Study. Circulation. 1994;90:2225–2229. doi: 10.1161/01.cir.90.5.2225. [DOI] [PubMed] [Google Scholar]
- 5.Carney RM, Freedland KE. Depression, mortality, and medical morbidity in patients with coronary heart disease. Biol Psychiatry. 2003;54:241–247. doi: 10.1016/s0006-3223(03)00111-2. [DOI] [PubMed] [Google Scholar]
- 6.Grippo AJ, Johnson AK. Biological mechanisms in the relationship between depression and heart disease. Neurosci Biobehav Rev. 2002;26:941–962. doi: 10.1016/s0149-7634(03)00003-4. [DOI] [PubMed] [Google Scholar]
- 7.Schleifer SJ, Macari-Hinson MM, Coyle DA, Slater WR, Kahn M, Gorlin R, et al. The nature and course of depression following myocardial infarction. Arch Int Med. 1989;149:1785–1789. [PubMed] [Google Scholar]
- 8.Freedland KE, Rich MW, Skala JA, Carney RM, Dávila-Román VG, Jaffe AS. Prevalence of depression in hospitalized patients with congestive heart failure. Psychosom Med. 2003;65:119–128. doi: 10.1097/01.psy.0000038938.67401.85. [DOI] [PubMed] [Google Scholar]
- 9.Grippo AJ, Francis J, Weiss RM, Felder RB, Johnson AK. Cytokine mediation of experimental heart failure-induced anhedonia. Am J Physiol Regul Integr Comp Physiol. 2003;284:R666–R673. doi: 10.1152/ajpregu.00430.2002. [DOI] [PubMed] [Google Scholar]
- 10.Sgoifo A, Koolhaas J, Alleva E, Musso E, Parmigiani S. Social stress: acute and long-term effects on physiology and behavior. Physiol Behav. 2001;73:253–254. doi: 10.1016/s0031-9384(01)00544-3. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs E, Flügge G. Chronic social stress: effects on limbic brain structures. Physiol Behav. 2003;79:417–427. doi: 10.1016/s0031-9384(03)00161-6. [DOI] [PubMed] [Google Scholar]
- 12.Tamashiro KLK, Nguyen MMN, Sakai RR. Social stress: from rodents to primates. Front Neuroendocrinol. 2005;26:27–40. doi: 10.1016/j.yfrne.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Grippo AJ, Moffitt JA, Johnson AK. Cardiovascular alterations and autonomic imbalance in an experimental model of depression. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1333–R1341. doi: 10.1152/ajpregu.00614.2001. [DOI] [PubMed] [Google Scholar]
- 14.Hilakivi LA, Ota M, Lister RG. Effects of isolation on brain monoamines and the behavior of mice in tests of exploration, locomotion, anxiety and behavioral ‘despair’. Pharmacol Biochem Behav. 1989;33:371–374. doi: 10.1016/0091-3057(89)90516-9. [DOI] [PubMed] [Google Scholar]
- 15.Grippo AJ, Cushing BS, Carter CS. Depression-like behavior and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosom Med. 2007;69:149–157. doi: 10.1097/PSY.0b013e31802f054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stilli D, Berni R, Sgoifo A, Costoli T, Bocchi L, Cacciani F, et al. Social stress, myocardial damage and arrhythmias in rats with cardiac hypertrophy. Physiol Behav. 2001;73:351–358. doi: 10.1016/s0031-9384(01)00454-1. [DOI] [PubMed] [Google Scholar]
- 17.Krantz DS, McCeney MK. Effects of psychological and social factors on organic disease: a critical assessment of research on coronary heart disease. Annu Rev Psychol. 2002;53:341–369. doi: 10.1146/annurev.psych.53.100901.135208. [DOI] [PubMed] [Google Scholar]
- 18.Thayer JF, Sternberg E. Beyond heart rate variability: vagal regulation of allostatic systems. Ann NY Acad Sci. 2006;1088:361–372. doi: 10.1196/annals.1366.014. [DOI] [PubMed] [Google Scholar]
- 19.Kiecolt-Glaser JK, Newton TL. Marriage and health: his and hers. Psychol Bull. 2001;127:472–503. doi: 10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- 20.Cacioppo JT, Hawkley LC, Crawford LE, Ernst JM, Burleson MH, Kowalewski RB, et al. Loneliness and health: potential mechanisms. Psychosom Med. 2002;64:407–417. doi: 10.1097/00006842-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Knox SS, Uvnäs-Moberg K. Social isolation and cardiovascular disease: an atherosclerotic pathway? Psychoneuroendocrinology. 1998;23:877–890. doi: 10.1016/s0306-4530(98)00061-4. [DOI] [PubMed] [Google Scholar]
- 22.Steptoe A, Owen N, Kunz-Ebrecht SR, Brydon L. Loneliness and neuroendocrine, cardiovascular, and inflammatory stress responses in middle-aged men and women. Psychoneuroendocrinology. 2004;29:593–611. doi: 10.1016/S0306-4530(03)00086-6. [DOI] [PubMed] [Google Scholar]
- 23.Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- 24.Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG. Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long-Evans rats. Pharmacol Biochem Behav. 2002;73:131–140. doi: 10.1016/s0091-3057(02)00781-5. [DOI] [PubMed] [Google Scholar]
- 25.Knardahl S, Sanders BJ, Johnson AK. Effects of adrenal demedullation on stress-induced hypertension and cardiovascular responses to acute stress. Acta Physiol Scand. 1988;133:477–483. doi: 10.1111/j.1748-1716.1988.tb08431.x. [DOI] [PubMed] [Google Scholar]
- 26.Johnson AK, Anderson EA. Stress and arousal. In: Cacioppo JT, Tassinary LG, editors. Principles of psychophysiology: physical, social, and inferential elements. Cambridge University Press; Cambridge: 1990. pp. 216–252. [Google Scholar]
- 27.Carney RM, Saunders RD, Freedland KE, Stein P, Rich MW, Jaffe AS. Association of depression with reduced heart rate variability in coronary artery disease. Am J Cardiol. 1995;76:562–564. doi: 10.1016/s0002-9149(99)80155-6. [DOI] [PubMed] [Google Scholar]
- 28.Krittayaphong R, Cascio WE, Light KC, Sheffield D, Golden RN, Finkel JB, et al. Heart rate variability in patients with coronary artery disease: differences in patients with higher and lower depression scores. Psychosom Med. 1997;59:231–235. doi: 10.1097/00006842-199705000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Pitzalis MV, Iacoviello M, Todarello O, Fioretti A, Guida P, Massari F, et al. Depression but not anxiety influences the autonomic control of heart rate after myocardial infarction. Am Heart J. 2001;141:765–771. doi: 10.1067/mhj.2001.114806. [DOI] [PubMed] [Google Scholar]
- 30.Kawachi I, Sparrow D, Vokonas PS, Weiss ST. Decreased heart rate variability in men with phobic anxiety. Am J Cardiol. 1995;75:882–885. doi: 10.1016/s0002-9149(99)80680-8. [DOI] [PubMed] [Google Scholar]
- 31.Grippo AJ, Beltz TG, Johnson AK. Behavioral and cardiovascular changes in the chronic mild stress model of depression. Physiol Behav. 2003;78:703–710. doi: 10.1016/s0031-9384(03)00050-7. [DOI] [PubMed] [Google Scholar]
- 32.Tapanainen JM, Thomsem PEB, Køber L, Torp-Pedersen C, Mäkikallio TH, Still A-M, et al. Fractal analysis of heart rate variability and mortality after an acute myocardial infarction. Am J Cardiol. 2002;90:347–352. doi: 10.1016/s0002-9149(02)02488-8. [DOI] [PubMed] [Google Scholar]
- 33.Ferrari R, Censi S, Mastrorilli F, Boraso A. Prognostic benefits of heart rate reduction in cardiovascular disease. Eur Heart J Suppl. 2003;5(Suppl G):G10–G14. [Google Scholar]
- 34.La Rovere MT, Pinna GD, Maestri R, Mortara A, Capomolla S, Febo O, et al. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation. 2003;107:565–570. doi: 10.1161/01.cir.0000047275.25795.17. [DOI] [PubMed] [Google Scholar]
- 35.Guzzetti S, La Rovere MT, Pinna GD, Maestri R, Borroni E, Porta A, et al. Different spectral components of 24 h heart rate variability are related to different modes of death in chronic heart failure. Eur Heart J. 2005;26:357–362. doi: 10.1093/eurheartj/ehi067. [DOI] [PubMed] [Google Scholar]
- 36.Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- 37.Porges SW. Orienting in a defensive world: mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology. 1995;32:301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- 38.Grippo AJ, Santos CM, Johnson RF, Beltz TG, Martins JB, Felder RB, et al. Increased susceptibility to ventricular arrhythmias in a rodent model of experimental depression. Am J Physiol Heart Circ Physiol. 2004;286:H619–H626. doi: 10.1152/ajpheart.00450.2003. [DOI] [PubMed] [Google Scholar]
- 39.Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci Biobehav Rev. 1995;19:303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- 40.Grippo AJ, Lamb DG, Carter CS, Porges SW. Cardiac regulation in the socially monogamous prairie vole. Physiol Behav. 2007;90:386–393. doi: 10.1016/j.physbeh.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev. 1992;16:525–534. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- 42.Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology. 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- 43.Muscat R, Willner P. Suppression of sucrose drinking by chronic mild unpredictable stress: a methodological analysis. Neurosci Biobehav Rev. 1992;16:507–517. doi: 10.1016/s0149-7634(05)80192-7. [DOI] [PubMed] [Google Scholar]
- 44.Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: the elevated plus maze 20 years on. Neurosci Biobehav Rev. 2005;29:1193–1205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 45.File SE. New strategies in the search for anxiolytics. Drug Des Deliv. 1990;5:195–201. [PubMed] [Google Scholar]
- 46.Cushing BS, Carter CS. Peripheral pulses of oxytocin increase partner preferences in female, but not male, prairie voles. Horm Behav. 2000;37:49–56. doi: 10.1006/hbeh.1999.1558. [DOI] [PubMed] [Google Scholar]
- 47.Carter CS, Witt DM, Schneider J, Harris ZL, Volkening D. Male stimuli are necessary for female sexual behavior and uterine growth in prairie voles (Microtus ochrogaster) Horm Behav. 1987;21:74–82. doi: 10.1016/0018-506x(87)90032-8. [DOI] [PubMed] [Google Scholar]
- 48.American Psychiatric Association . Diagnostic and statistical manual of mental disorders, fourth edition, text revision. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 49.Konkle AT, Baker SL, Kentner AC, Barbagallo LS, Merali Z, Bielajew C. Evaluation of the effects of chronic mild stressors on hedonic and physiological responses: sex and strain compared. Brain Res. 2003;992:227–238. doi: 10.1016/j.brainres.2003.08.047. [DOI] [PubMed] [Google Scholar]
- 50.Sgoifo A, De Boer SF, Buwalda B, Korte-Bouws G, Tuma J, Bohus B, et al. Vulnerability to arrhythmias during social stress in rats with different sympathovagal balance. Am J Physiol Heart Circ Physiol. 1998;275:H460–H466. doi: 10.1152/ajpheart.1998.275.2.H460. [DOI] [PubMed] [Google Scholar]
- 51.Sgoifo A, Stilli D, Medici D, Gallo P, Aimi B, Musso E. Electrode positioning for reliable telemetry ECG recordings during social stress in unrestrained rats. Physiol Behav. 1996;60:1397–1401. doi: 10.1016/s0031-9384(96)00228-4. [DOI] [PubMed] [Google Scholar]
- 52.Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm enteries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 53.Bosch OJ, Krömer SA, Brunton PJ, Neumann ID. Release of oxytocin in the hypothalamic paraventricular nucleus, but not central amygdala or lateral septum in lactating residents and virgin intruders during maternal defence. Neuroscience. 2004;124:439–448. doi: 10.1016/j.neuroscience.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 54.Mitchell PJ, Fairhall SJ, Fletcher A, Redfern PH. Effects of single and repeated electroconvulsive shock on the social and agonistic behaviour of resident rats. Neuropharmacology. 2003;44:911–925. doi: 10.1016/s0028-3908(03)00075-3. [DOI] [PubMed] [Google Scholar]
- 55.Ishii K, Kuwahara M, Tsubone H, Sugano S. Autonomic nervous function in mice and voles (Microtus arvalis): investigation by power spectral analysis of heart rate variability. Lab Animals. 1996;30:359–364. doi: 10.1258/002367796780739880. [DOI] [PubMed] [Google Scholar]
- 56.Task Force of the European Society of Cardiology, North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 57.Yongue BG, McCabe PM, Porges SW, Rivera M, Kelley SL, Ackles PK. The effects of pharmacological manipulations that influence vagal control of the heart on heart period, heart-period variability and respiration in rats. Psychophysiology. 1982;19:426–432. doi: 10.1111/j.1469-8986.1982.tb02499.x. [DOI] [PubMed] [Google Scholar]
- 58.Porges SW, McCabe PM, Yongue BG. Respiratory-heart rate interactions: psychophysiological implications for pathophysiology and behavior. In: Cacioppo J, Petty R, editors. Perspectives in cardiovascular psychophysiology. Guilford Publications, Inc.; New York: 1982. pp. 223–264. [Google Scholar]
- 59.Porges SW. The polyvagal perspective. Biol Psychol. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gehrmann J, Hammer PE, Maguire CT, Wakimoto H, Triedman JK, Berul CI. Phenotypic screening for heart rate variability in the mouse. Am J Physiol Heart Circ Physiol. 2000;279:H733–H740. doi: 10.1152/ajpheart.2000.279.2.H733. [DOI] [PubMed] [Google Scholar]
- 61.Francis J, Weiss RM, Wei SG, Johnson AK, Felder RB. Progression of heart failure after myocardial infarction in the rat. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1734–R1745. doi: 10.1152/ajpregu.2001.281.5.R1734. [DOI] [PubMed] [Google Scholar]
- 62.Solberg LC, Horton TH, Turek FW. Circadian rhythms and depression: effects of exercise in an animal model. Am J Physiol Regul Integr Comp Physiol. 1999;276:R152–R161. doi: 10.1152/ajpregu.1999.276.1.R152. [DOI] [PubMed] [Google Scholar]
- 63.Bosch OJ, Nair HP, Neumann ID, Young LJ. Pair-bonded prairie voles display depression-like behavior after separation. Soc Neurosci Abstr. 2004 http://sfn.scholarone.com/itin2004/main.html?new_page_id=126&abstract_id=7974&p_num=762.12&is_tech=0. [Google Scholar]
- 64.Willner P, Moreau J-L, Nielsen CK, Papp M, Sluzewska A. Decreased hedonic responsiveness following chronic mild stress is not secondary to loss of body weight. Physiol Behav. 1996;60:129–134. doi: 10.1016/0031-9384(95)02256-2. [DOI] [PubMed] [Google Scholar]
- 65.Lovallo WR. Cardiovascular reactivity: mechanisms and pathways to cardiovascular disease. Int J Psychophysiol. 2005;58:119–132. doi: 10.1016/j.ijpsycho.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 66.Tafet GE, Bernardini R. Psychoneuroendocrinological links between chronic stress and depression. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:893–903. doi: 10.1016/S0278-5846(03)00162-3. [DOI] [PubMed] [Google Scholar]
- 67.Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. Am J Psychiatry. 1992;149:999–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- 68.Kjekshus JK, Blix AS, Grottum P, Aasen AO. Beneficial effects of vagal stimulation on the ischaemic myocardium during beta-receptor blockade. Scand J Clin Lab Invest. 1981;41:383–389. doi: 10.3109/00365518109092060. [DOI] [PubMed] [Google Scholar]
- 69.Lown B, Verrier RL. Neural activity and ventricular fibrillation. N Engl J Med. 1976;294:1165–1170. doi: 10.1056/NEJM197605202942107. [DOI] [PubMed] [Google Scholar]
- 70.Kleiger RE, Miller JP, Bigger JT, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59:256–262. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- 71.Kristal-Boneh E, Raifel M, Froom P, Ribak J. Heart rate variability in health and disease. Scand J Work Environ Health. 1995;21:85–95. doi: 10.5271/sjweh.15. [DOI] [PubMed] [Google Scholar]


