Abstract
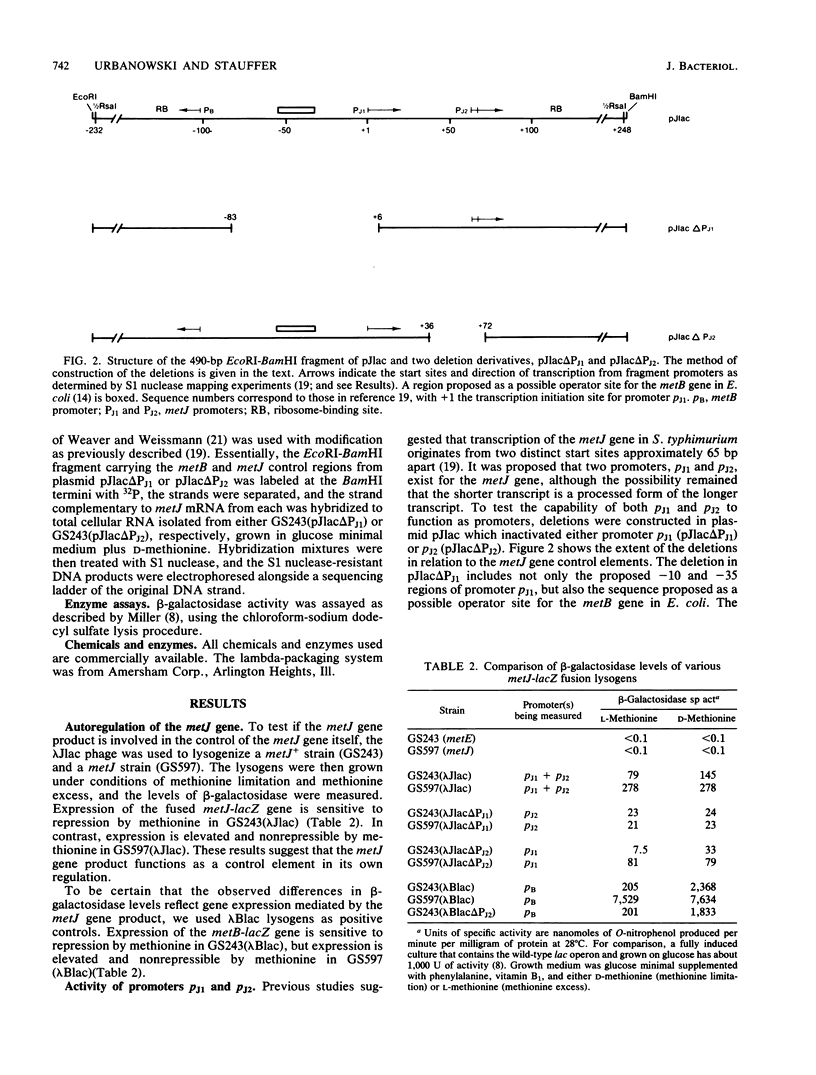
Regulation of the Salmonella typhimurium metJ gene was examined by measuring beta-galactosidase activity in Escherichia coli strains lysogenic for a phage carrying a metJ-lacZ gene fusion. The results indicated that the metJ gene is regulated by its own gene product and by methionine supplementation to the growth medium. This autoregulatory mechanism involved two tandem promoters, pJ1 and pJ2, separated by approximately 65 base pairs. Deletion analysis permitted the assessment of the activity of promoters pJ1 and pJ2 individually. Promoter Pj1 was negatively regulated by the metJ gene product and by methionine. Although Pj2 regulation remained unclear, evidence is presented which suggests that it is not negatively regulated like pJ1.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brent R., Ptashne M. The lexA gene product represses its own promoter. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1932–1936. doi: 10.1073/pnas.77.4.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchange N., Zakin M. M., Ferrara P., Saint-Girons I., Park I., Tran S. V., Py M. C., Cohen G. N. Structure of the metJBLF cluster in Escherichia coli K12. Sequence of the metB structural gene and of the 5'- and 3'-flanking regions of the metBL operon. J Biol Chem. 1983 Dec 25;258(24):14868–14871. [PubMed] [Google Scholar]
- Kelley R. L., Yanofsky C. Trp aporepressor production is controlled by autogenous regulation and inefficient translation. Proc Natl Acad Sci U S A. 1982 May;79(10):3120–3124. doi: 10.1073/pnas.79.10.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence D. A., Smith D. A., Rowbury R. J. Regulation of methionine synthesis in Salmonella typhimurium: mutants resistant to inhibition by analogues of methionine. Genetics. 1968 Apr;58(4):473–492. doi: 10.1093/genetics/58.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso R. E., Di Lauro R., Adhya S., de Crombrugghe B. Dual control for transcription of the galactose operon by cyclic AMP and its receptor protein at two interspersed promoters. Cell. 1977 Nov;12(3):847–854. doi: 10.1016/0092-8674(77)90283-5. [DOI] [PubMed] [Google Scholar]
- Panasenko S. M., Cameron J. R., Davis R. W., Lehman I. R. Five hundredfold overproduction of DNA ligase after induction of a hybrid lambda lysogen constructed in vitro. Science. 1977 Apr 8;196(4286):188–189. doi: 10.1126/science.322281. [DOI] [PubMed] [Google Scholar]
- Piette J., Nyunoya H., Lusty C. J., Cunin R., Weyens G., Crabeel M., Charlier D., Glansdorff N., Piérard A. DNA sequence of the carA gene and the control region of carAB: tandem promoters, respectively controlled by arginine and the pyrimidines, regulate the synthesis of carbamoyl-phosphate synthetase in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4134–4138. doi: 10.1073/pnas.81.13.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitzer L. J., Magasanik B. Expression of glnA in Escherichia coli is regulated at tandem promoters. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1979–1983. doi: 10.1073/pnas.82.7.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Girons I., Duchange N., Cohen G. N., Zakin M. M. Structure and autoregulation of the metJ regulatory gene in Escherichia coli. J Biol Chem. 1984 Nov 25;259(22):14282–14285. [PubMed] [Google Scholar]
- Smith A. A., Greene R. C., Kirby T. W., Hindenach B. R. Isolation and characterization of the product of the methionine-regulatory gene metJ of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6104–6108. doi: 10.1073/pnas.82.18.6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer G. V., Plamann M. D., Stauffer L. T. Construction and expression of hybrid plasmids containing the Escherichia coli glyA genes. Gene. 1981 Jun-Jul;14(1-2):63–72. doi: 10.1016/0378-1119(81)90148-7. [DOI] [PubMed] [Google Scholar]
- Su C. H., Greene R. C. Regulation of methionine biosynthesis in Escherichia coli: mapping of the metJ locus and properties of a metJ plus-metJ minus diploid. Proc Natl Acad Sci U S A. 1971 Feb;68(2):367–371. doi: 10.1073/pnas.68.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
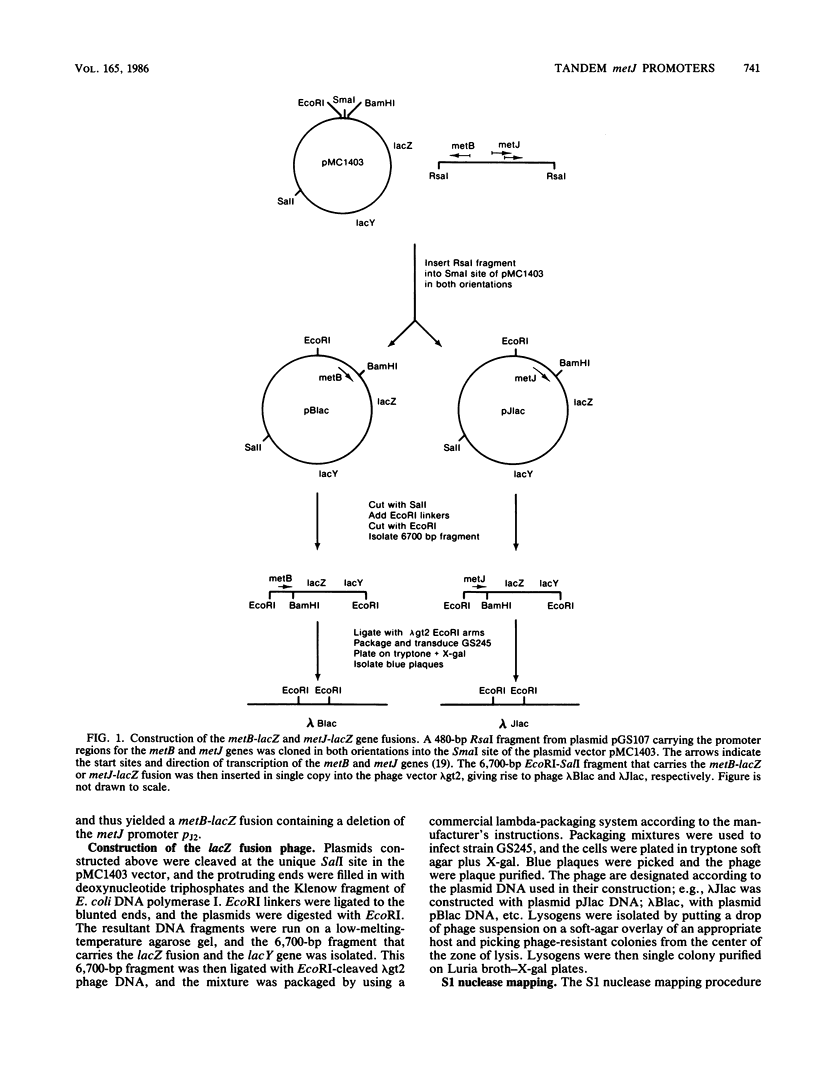
- Urbanowski M. L., Stauffer G. V. Cloning and initial characterization of the metJ and metB genes from Salmonella typhimurium LT2. Gene. 1985;35(1-2):187–197. doi: 10.1016/0378-1119(85)90171-4. [DOI] [PubMed] [Google Scholar]
- Urbanowski M. L., Stauffer G. V. Nucleotide sequence and biochemical characterization of the metJ gene from Salmonella typhimurium LT2. Nucleic Acids Res. 1985 Feb 11;13(3):673–685. doi: 10.1093/nar/13.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. G., Lee N., Fowler A. V. The araC gene of Escherichia coli: transcriptional and translational start-points and complete nucleotide sequence. Gene. 1980 Dec;12(3-4):179–190. doi: 10.1016/0378-1119(80)90100-6. [DOI] [PubMed] [Google Scholar]
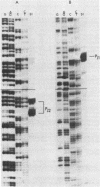
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil J., Cunningham R., Martin R., 3rd, Mitchell E., Bolling B. Characteristics of lambda p4, a lambda derivative containing 9 per cent excess DNA. Virology. 1972 Nov;50(2):373–380. doi: 10.1016/0042-6822(72)90388-1. [DOI] [PubMed] [Google Scholar]