Abstract
ErbB receptors are important regulators of fetal organ development, including the fetal lung. They exhibit diversity in signaling potential, acting through homo- and heterodimers to cause different biological responses. We hypothesized that ErbB receptors show cell-specific and stimuli-specific, activation, heterodimerization, and cellular localization patterns in fetal lung. We investigated this using immunoblotting, co-immunoprecipitation, and confocal microscopy in primary isolated E19 fetal rat lung fibroblasts and epithelial type II cells, stimulated with epidermal growth factor, transforming growth factor α, neuregulin 1β or treated with conditioned medium (CM) from the respective other cell type. Fetal type II cells expressed significantly more ErbB1, ErbB2, and ErbB3 protein than fibroblasts. ErbB4 was consistently identified by co-immunoprecipitation of all other ErbB receptors in both cell types independent of the treatments. Down regulation of ErbB4 in fibroblasts initiated cell-cell communication that stimulated surfactant phospholipid synthesis in type II cells. Confocal microscopy in type II cells revealed nuclear localization of all receptors, most prominently for ErbB4. Neuregulin treatment resulted in relocation to the extra-nuclear cytoplasmic region, which was distinct from fibroblast CM treatment which led to nuclear localization of ErbB4 and ErbB2, inducing co-localization of both receptors. We speculate that ErbB4 plays a prominent role in fetal lung mesenchyme-epithelial communication.
Keywords: Neuregulin, epidermal growth factor, transforming growth factor α, conditioned medium, lung development
Introduction
The four members of the ErbB receptor family (epidermal growth factor receptor (EGFR)/ErbB1, ErbB2, ErbB3 and ErbB4) are transmembrane tyrosine kinases with structural homologies [32]. ErbB receptor signaling leads to a diversity of biologic effects during embryonic development. Through interaction with each other the receptors form an array of homo-and heterodimers leading to signal diversification that permits ErbB receptors to control developmental processes in diverse tissues [1]. The importance of ErbB receptors in development has been demonstrated in knockout mouse models for each receptor. Loss of ErbB1 leads to an overall pulmonary epithelial immaturity, including impaired branching, deficient alveolarization, reduced surfactant protein, and a marked reduction in alveolar volume in the lung [21]. ErbB2 null mice die at mid-gestation (E10.5) due to central nervous system and cardiac malformations [18], a phenotype that is shared by ErbB4 knockout mice [12]. Most ErbB3 knock out mice die by E13.5 and have gross malformations of the nervous system and Schwann cells. A few embryos survive to birth but die shortly afterwards of respiratory insufficiency and exhibit uninflated lungs [33]. The early embryonic lethality of the ErbB knockout models precludes developing any information on ErbB receptor involvement in late gestation lung maturation.
ErbB ligands are involved in lung maturation. The mRNA transcripts of epidermal growth factor (EGF) and transforming growth factor-alpha (TGFα) are expressed in fetal lung mesenchymal cells [35]. EGF and TGFα, the ligands for ErbB1, positively influence surfactant synthesis through mesenchymal-epithelial cell communication [24,25], whereas neuregulin (NRG)1β, the ligand for ErbB3 and ErbB4 [4], is secreted by fetal lung fibroblasts and stimulates surfactant synthesis directly in the fetal type II cell [8]. This stimulatory effect can be inhibited by antibodies to NRG1β [8] and ErbB receptors [22]. NRG1β, particularly when its secretion is induced by glucocorticoids, stimulates ErbB2 receptor phosphorylation in epithelial type II cells [8]. Because ErbB2 has no known ligand it must act as a heterodimer partner of ligand-bound ErbB1, ErbB3, or ErbB4. The activation of ErbB2 by NRG1β in type II cells suggests heterodimerization of ErbB2 with ErbB3 or ErbB4.
Little is known about ErbB receptor dimerization in fetal lung fibroblasts and type II cells. We hypothesized that specific ErbB receptor dimers are significant for fibroblast–type II cell communication that controls late gestation lung development. We therefore studied ErbB receptor protein content, dimerization, phosphorylation, and co-localization patterns in primary cultures of fetal E19 rat lung fibroblasts and alveolar epithelial type II cells. Our experiments were done in untreated cells as well as in EGF-, transforming growth factor alpha (TGFα)0, NRG1β-, fibroblast conditioned medium (FCM)-, and type II cell conditioned medium (T2CM)-treated cells. We also used siRNA to down regulate ErbB4 in fetal lung fibroblasts to study the effect on fibroblast-type II cell communication.
Materials and Methods
Timed-pregnant Sprague-Dawley rats were obtained from Taconic Farms (Germantown, NY); Dulbecco’s modified eagle’s medium (DMEM), Hanks balanced salt solution (HBSS), and DNAse were from Gibco (Grand Island, NY); plastic tissue culture dishes, flasks and six-well tissue culture plates were from Falcon/Becton Dickinson (Franklin Lakes, NJ); charcoal-stripped fetal calf serum (FCS) was from Hyclone (Logan, UT); mouse monoclonal anti-actin clone AC-40, epidermal growth factor (EGF), and TGFα were from Sigma (St. Louis, MO); ErbB4 siRNAs, scrambled siRNA, GAPDH siRNA and mouse anti GAPDH antibody were from Ambion (Austin, TX); transfection reagent TKO was from Mirus (Madison, WI); HRP-labeled goat anti-rabbit IgG and goat anti-mouse IgG were from Perkin Elmer Life Sciences, Inc (Boston, MA); rabbit polyclonal IgG antibodies to EGFR (1005), ErbB-3 (C-17), and ErbB-4 (C-18), SK-BR-3 cell lysate and Blotto were from Santa Cruz Biotechnology (Santa Cruz, CA); rabbit polyclonal antibody c-erbB-2 (Ab-1), mouse monoclonal antibodies c-erbB-2 (Ab-17), and c-erbB-3 (Ab-6) were from LabVision (Fremont, CA); rabbit polyclonal IgG antibody was from Zymed (South San Francisco, CA); protein A Sepharose™ CL-4B was from Amersham Biosciences (Uppsala, Sweden); Precision Plus Protein™ standards (dual color) and nitrocellulose membranes were from Bio-Rad Laboratories (Hercules, CA); recombinant horseradish peroxidase-linked anti-phosphotyrosine antibody RC20 was from Transduction Laboratories (Palo Alto, CA); NRG1β producer cells were a generous gift from Dr. Kermit Carraway, III, (UC Davis, CA). NRG1β was purified from cultured cells by Dr. Ann Kane of the Center for Gastroenterology Research in Absorptive and Secretory Processes (GRASP) at Tufts–New England Medical Center.
Fetal rat lung fibroblast and epithelial type II cell cultures
The animal research protocol was approved by the institutional IACUC. Pregnant rats were sacrificed by CO2 inhalation at E19 of gestation (term E22). The fetuses were removed and sex determined [26]. The lungs were removed, pooled according to sex, minced into 1mm3 pieces and dissociated in Hanks buffered saline solution (HBSS) containing DNAse (20 μg/ml) and 0.25% trypsin at 37°C for 10 minutes. The dissociation was stopped by adding DMEM with 10% charcoal stripped fetal calf serum (FCS−). The cells were filtered through a sterile 45-μm cell strainer and centrifuged at 650-x g for 10 minutes at 4°C. The cell pellets were resuspended in DMEM with 10% FCS− and total cells were plated at 6 × 106/dish in 100mm2 culture dishes for 60 minutes at 37°C to allow for lung fibroblast adherence.
For type II cell cultures, medium containing the non-adherent cells was removed, pooled from both sexes, and centrifuged at 650-x g for 10 minutes at 4°C. Cell pellets were resuspended in DMEM with 20% FCS−. The cells were plated in 125 mm2 flasks and incubated for 60 minutes at 37°C to allow a second differential adherence. The non-adherent cells were collected, centrifuged as described above, resuspended in DMEM with 20% FCS−, and plated into 12 well tissue culture plates (4 × 106 cells/well) or 6 well plates (6 × 106 cells/well) and cultured in 37°C, 5% CO2 incubator overnight. The non-adherent cells were washed away with DMEM. The purity of the type II cells was determined by methods of Post and Smith [29], preparations with greater than 92% purity were used for experiments.
Fibroblast and epithelial type II cell conditioned medium
When fibroblasts or type II cells reached 90% confluence the cells were serum starved for 24 hours in serum-free medium. This medium was collected from fibroblasts as fibroblast conditioned medium (FCM) and from type II cells as type II cell conditioned medium (T2CM).
siRNA experiments
The fibroblasts were plated in 6 well plates at 15×104/well or 24 well plates at 3.5×104/well to obtain 50% confluence next day. Three different ErbB4 siRNAs which target the rat ErbB4 mRNA sequences 404-422 (site 1), 661-680 (site 2) and 2063-2081 (site 3) were used (Table 1), together with the transfection reagent Transit-TKO. Scrambled siRNA was used for a nonspecific control. The initial transfection lasted 48 hours. siRNAs were then added with serum free medium for the last 24 hours and the media collected as FCM that was used to treat type II cells at the 1:1 ratio to DMEM.
Table 1.
Rat ErbB4 siRNA sequences.
| ErbB4 siRNA | Target rat ErbB4 mRNA sites | Sense sequences 5′-3′ | Anti-sense Sequences 5′-3′ |
|---|---|---|---|
| Site 1 | 404-422 | GGAAAGAUGGCAACUUUGGtt | CCAAAGUUGCCAUCUUUCCtg |
| Site 2 | 661-680 | GGACUGUGUGUGCAGAACAtt | UGUUCUGCACACACAGUCCtt |
| Site 3 | 2063-2081 | GGAAGAGCAUCAAAAAGAAtt | UUCUUUUUGAUGCUCUUCCtt |
3H-Choline incorporation
Confluent type II cell cultures were treated with 0.5 μCi 3H-choline for 24 hours. Cells were washed 3 times with ice cold PBS, scraped in PBS and sonicated. Aliquots in duplicate were used for protein determination. Following lipid extraction with chloroform and methanol, Disaturated phosphatidylcholine (DSPC) was isolated by osmium tetroxide and separated by thin-layer chromatography on silica gel H chromatography sheets. The resulting spots were scraped into scintillation fluid, counted in a beta scintillation counter, expressed as disintegrations per min per μg protein, and normalized as percent of experiment specific controls.
Co-immunoprecipitation (co-IP) and Western blotting of the ErbB receptors
The cells were stimulated with EGF (17nM) for 3 minutes, TGFα (18nM) for 5 minutes, NRG1β (33nM) for 2 minutes, or serum-free DMEM (controls) for 3 minutes. Alternatively, fibroblasts were exposed for 24 hours to T2CM, and type II cells were exposed for the same time to FCM. The cells were washed with ice cold PBS and lysed in co-IP buffer (20mM Tris (pH 7.4), 150mM NaCl, 1 mM MgCl2, 0.1% Triton X-100, 10% glycerol, 1mM Na3VO4, 1 mM NaF, 1mM ZnCl2, 10 mM β-glycerol phosphate, 5mM tetrasodium pyrophosphate, 1 mM phenylmethylsulfonyl fluoride, and 4 μg/ml each of aprotinin, leupeptin and pepstatin). Duplicate aliquots were used for protein determination [20]. 500 μg of total fibroblast protein or 300 μg of total type II cell protein were incubated for 90 minutes at 4°C with 2μg of the specific receptor antibody (anti-EGFR 1005, anti-ErbB2 Ab1, anti-ErbB3 C17, anti-ErbB4 C18, or rabbit polyclonal IgG). Protein-A-Sepharose was added and the incubation was continued at 4°C overnight. The beads were collected by microcentrifugation at 100 × g and 4°C for 30 seconds, washed three times with co-IP washing buffer (20mM HEPES (pH 7.4), 150mM NaCl, 1 mM EDTA, 0.1% Triton X-100, 1mM Na3VO4, 1 mM NaF, 10 mM β-glycerol phosphate, 5 mM tetrasodium pyrophosphate, 0.2 mM phenylmethylsulfonyl fluoride, and 4 μg/ml each of aprotinin, leupeptin, and pepstatin), and boiled in Laemmli sample loading buffer for 5 minutes at 100°C. The immunoprecipitated protein and paired lysates were separated by 7% SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Ponceau staining was used to confirm equal protein loading. The blots were blocked in 1% BSA and incubated with recombinant horseradish peroxidase-linked anti-phosphotyrosine antibody RC20 (1:5000) overnight at 4°C, washed three times with TBST, and bands visualized by enhanced chemiluminescence. Membranes were stripped in stripping buffer (62.5 mM Tris (pH 6.8), 2% SDS, 0.8% beta-mercaptoethanol) for 30 minutes at 50°C, blocked with 5% non-fat milk and reprobed with an antibody against one of the other ErbB receptors (EGFR Ab1005, ErbB2 Ab17, ErbB3 Ab6, or ErbB4 C18), or actin Ab (clone AC-40) overnight at 4°C. Appropriate secondary antibody was applied and bands were visualized by enhanced chemiluminescence. Membranes were stripped for reprobing up to a maximum of 4–5 times. Densitometry was performed, and the readings for bands of phosphorylation were divided by the total receptor protein to determine activation per receptor amount.
Confocal Microscopy
Primary rat type II cells were cultured on glass cover slips. After 24h serum starvation, cells were treated with FCM, NRG1β (33nM) or DMEM (controls) for 24h. Immunofluorescence was done as described [28]. Cells were rinsed with DMEM, fixed for 20 minutes in 3% paraformaldehyde and permeabilized for 2 minutes with 0.2% Triton X-100 in PBS. After blocking in 10% normal goat serum, cells were incubated with primary ErbB-antibody (EGFR Ab1005, ErbB2 Ab15, ErbB2 Ab1, ErbB3 C17, ErbB3 Ab5, ErbB4 C7) for 30 minutes at room temperature, washed with PBS and incubated with secondary antibody (Alexa Fluor 488, Alexa Fluor 568) for 30 minutes at room temperature. Cells were mounted in Gelvatol/DABCO and analyzed using a Leica TCS – SP2 confocal laser scanning microscope.
Data analysis
Data are presented as Mean±SEM unless otherwise stated. Densitometry was used to quantitatively analyze Western blots of IP’s. Statistical analyses were performed using either paired two-sample t-tests or one way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test to correct for multiple comparisons of individual treatments versus control (Instat Statistical Package, Graphpad Software Inc., San Diego, CA).
Results
Expression pattern of the four ErbB receptors
All four ErbB receptors were expressed in fetal lung fibroblasts and type II cells (Fig 1A.). The Western blots of ErbB receptors were quantitated by densitometry and normalized to β actin to correct for loading variability. Densitometry of female and male lung fibroblasts identified no differences in receptor protein amount for all four receptors. We therefore combined the quantitative data from both sexes. Compared to the expression in fibroblasts, three of four ErbB receptors appeared more abundant in the fetal type II cells. This was statistically significant for ErbB1, ErbB2, and ErbB3. ErbB1 receptor protein was 68±22 densitometry units (units) in type II cells compared to 51±16 units in fibroblasts (N=7, p=0.0005), ErbB2 was 61±15 units in type II cells and 39±8.7 units in fibroblasts (N=7, p=0.009), ErbB3 was 93±9 units in type II cells and 41±9 units in fibroblasts (N=7, p=0.002), ErbB4 was 42±11 units in type II cells and 46±14 units in fibroblasts (N=7, p=0.18) (Fig. 1B), revealing cell specific ErbB receptor expression patterns.
Fig 1. ErbB receptors in rat fetal lung fibroblasts and alveolar epithelial type II cells.

A: Representative blots of phosphorylated (upper panel) and total ErbB1, ErbB2, ErbB3 and ErbB4 (middle panels) and actin (lower panel) in female fibroblasts (left side) and alveolar type II cells (right side). B: Densitometric analysis of ErbB receptor protein amounts. Values represent the ratio of receptor protein to β actin, Mean±SEM, N=7, *: P<0.05.
ErbB receptor heterodimerization and phosphorylation in response to growth factors in E19 fetal rat lung fibroblasts and epithelial type II cells
Co-immunoprecipitation (Co-IP) was used to determine the heterodimers formed in the fetal rat lung fibroblasts and type II cells and the phosphorylation of dimer members at baseline and in response to stimulation with different ErbB ligands. The signals were quantified by densitometry. The growth factor-induced phosphorylated proportion of total ErbB protein was compared to that of the untreated cells. To exclude non-specific binding, antibody to IgG was also used for Co-IP. All blots were probed with antibodies to phosphotyrosine and reprobed for each ErbB receptor. Co-IP with the irrelevant IgG antibody did not reveal any ErbB signals, demonstrating the specificity of our ErbB Co-IP results (Fig 2i).
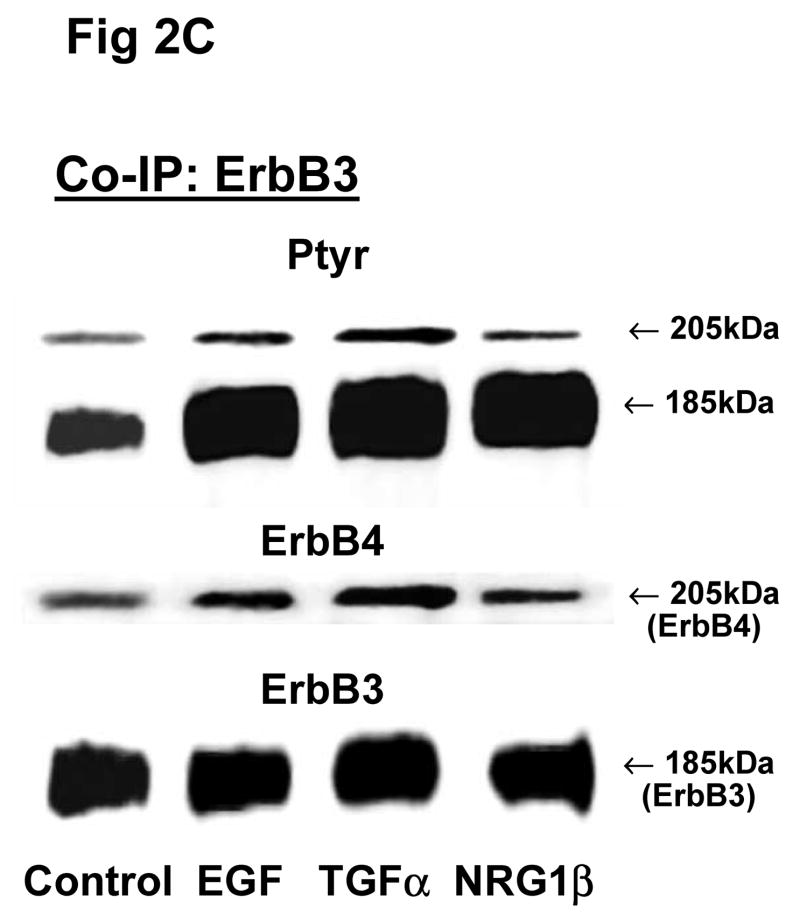
Fig 2. Co-immunoprecipitation of ErbB receptor heterodimers in response to growth factor stimulations in rat fetal lung fibroblasts and alveolar epithelial type II cells.

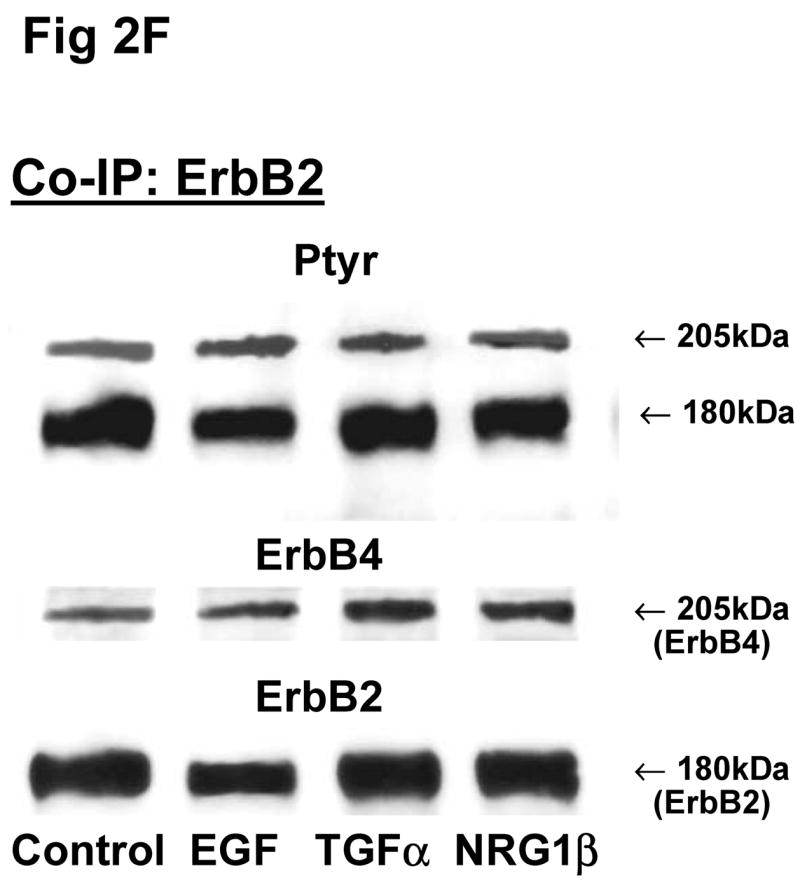
Cells were unstimulated (control) or stimulated with EGF, TGFα, or NRG1β and immunoprecipitated with antibody to ErbB1 (A, E), ErbB2 (B, F), ErbB3 (C, G), ErbB4 (D, H) or IgG (I) under co-immunoprecipitation conditions. Panels show representative blots for fibroblasts (A, B, C, D and I) and type II cells (E, F, G, H); illustrated results were consistently found in 4–5 independent experiments.
Since there were no significant differences in receptor protein amounts between male and female fetal lung fibroblasts, we used female fibroblasts for the Co-IP experiments. In female fibroblasts, antibody to ErbB1 co-immunoprecipitated ErbB4 (Fig 2A). Compared to untreated fibroblasts, stimulation with EGF increased the phosphorylated proportion of both precipitated ErbB1 (301±22%, N=5, P<0.01) and co-precipitated ErbB4 (187±22%, N=5, P<0.01), while TGFα increased ErbB1 phosphorylation proportion (235±34%, N=5, P<0.01) and co-immunoprecipitated ErbB4 (165±12%, N=5, P<0.05) (Table 2). Antibody to ErbB2 co-immunoprecipitated ErbB4. The co-precipitated ErbB4 had a significant increase in phosphorylation proportion after EGF treatment (234±49%, N=5, P<0.01), when compared to untreated cells (Fig. 2B; Table 2). Antibody to ErbB3 co-precipitated ErbB4 (Fig 2C). Precipitated ErbB3 phosphorylation proportion was significantly increased by EGF (196±38%, N=5, P<0.05). Surprisingly, Co-IP with antibody to ErbB4 only revealed immunoprecipitation of ErbB4. No other ErbB receptor was detected on Western blot. EGF, TGFα and NRG1β each slightly stimulated phosphorylation proportion of the precipitated ErbB4 receptor, but this was not statistically significant (Fig 2D; Table 2).
Table 2. Co-IP Densitometry of phosphorylated signals of ErbB dimers.
Values are percentage of treatment phosphorylation signals over the receptor proteins and then normalized to the experiment control, Mean±SEM. (N=4–5 experiments).
| EGF | TGFα | NRG1β | ANOVA | |
|---|---|---|---|---|
| FIBROBLASTS | ||||
| Co-IP ErbB1 | ||||
| ErbB4 phosphorylation | 187±22** | 165±12* | 116±17 | P=0.001 |
| ErbB1 phosphorylation | 301±32** | 235±34** | 157±12 | P<0.0001 |
| Co-IP ErbB2 | ||||
| ErbB4 phosphorylation | 234 ±49** | 126±12 | 99±11 | P=0.001 |
| ErbB2 phosphorylation | 176±33 | 116±8 | 123±25 | P=0.13 |
| Co-IP ErbB3 | ||||
| ErbB4 phosphorylation | 192±44 | 166±32 | 142±3 | P=0.27 |
| ErbB3 phosphorylation | 196±38* | 137±15 | 127±18 | P=0.21 |
| Co-IP ErbB4 | ||||
| ErbB4 phosphorylation | 161±30 | 172±41 | 131±15 | P=0.09 |
| TYPE II CELLS | ||||
| Co-IP ErbB1 | ||||
| ErbB4 phosphorylation | 199±68 | 137±15 | 127±18 | P=0.21 |
| ErbB2 phosphorylation | 308±52 | 172±47 | 170±50 | P=0.15 |
| ErbB1 phosphorylation | 118±17 | 105±4 | 89±21 | P=0.58 |
| Co-IP ErbB2 | ||||
| ErbB4 phosphorylation | 115±4* | 87±3 | 79±5** | P<0.0001 |
| ErbB2 phosphorylation | 109±8 | 110±9 | 97±12 | P=0.34 |
| Co-IP ErbB3 | ||||
| ErbB4 phosphorylation | 166±41 | 119±22 | 133±2 | P=0.57 |
| ErbB2/3 phosphorylation | 117±7 | 115±9 | 141±19 | P=0.22 |
| Co-IP ErbB4 | ||||
| ErbB4 phosphorylation | 103±28 | 103±28 | 102±2 | P=0.99 |
| ErbB1 phosphorylation | 109±11 | 116±22 | 123±11 | P=0.36 |
P value for the ANOVA is shown.
=P<0.05,
P<0.001 compared to unstimulated control, Dunnett multiple comparison test.
ErbB4 receptor was co-immunoprecipitated with ErbB1, ErbB2 and ErbB3 antibodies in fetal type II cells (Fig 2E, 2F, 2G). Fetal type II cells differed from fibroblasts in their Co-IP patterns in two ways. First, antibody to ErbB1 and to ErbB3 each co-precipitated ErbB2 in addition to ErbB4 (Fig 2E, 2G). Second, antibody to ErbB4 co-precipitated ErbB1 (Fig 2H). When type II cells were stimulated with EGF, TGFα, or NRG1β, there was a smaller stimulation of the phosphorylated proportion of both the primary receptor and the co-precipitated dimers (Table 2).
These Co-IP results for both fibroblasts and type II cells were found in each of 5 and 3–4 separate experiments respectively, and in no instance did we observe a heterodimer partner in fewer than this number of separate experiments. Table 3 summarizes the ErbB receptor dimerization patterns for female fetal lung fibroblasts and type II cells. Overall, ErbB4 appeared to be the common heterodimer partner in both fibroblasts and type II epithelial cells of the fetal rat lung. Table 2 summarizes the densitometry analysis of phosphorylation of the co-precipitated ErbB receptor dimers in response to stimulation by EGF, TGFα and NRG1β. Analysis of phosphorylation responses by ANOVA confirmed the reproducibility of finding specific co-IP partners.
Table 3.
Summary of major heterodimers of ErbB receptors in fetal lung cells.
| Fibroblasts | Type II cells | |
|---|---|---|
| ErbB1 | ErbB4 | ErbB4, ErbB2 |
| ErbB2 | ErbB4 | ErbB4 |
| ErbB3 | ErbB4 | ErbB4, ErbB2 |
| ErbB4 | - | ErbB1 |
ErbB receptor heterodimerization and phosphorylation in fibroblast-epithelial type II cell communication in E19 fetal rat lungs
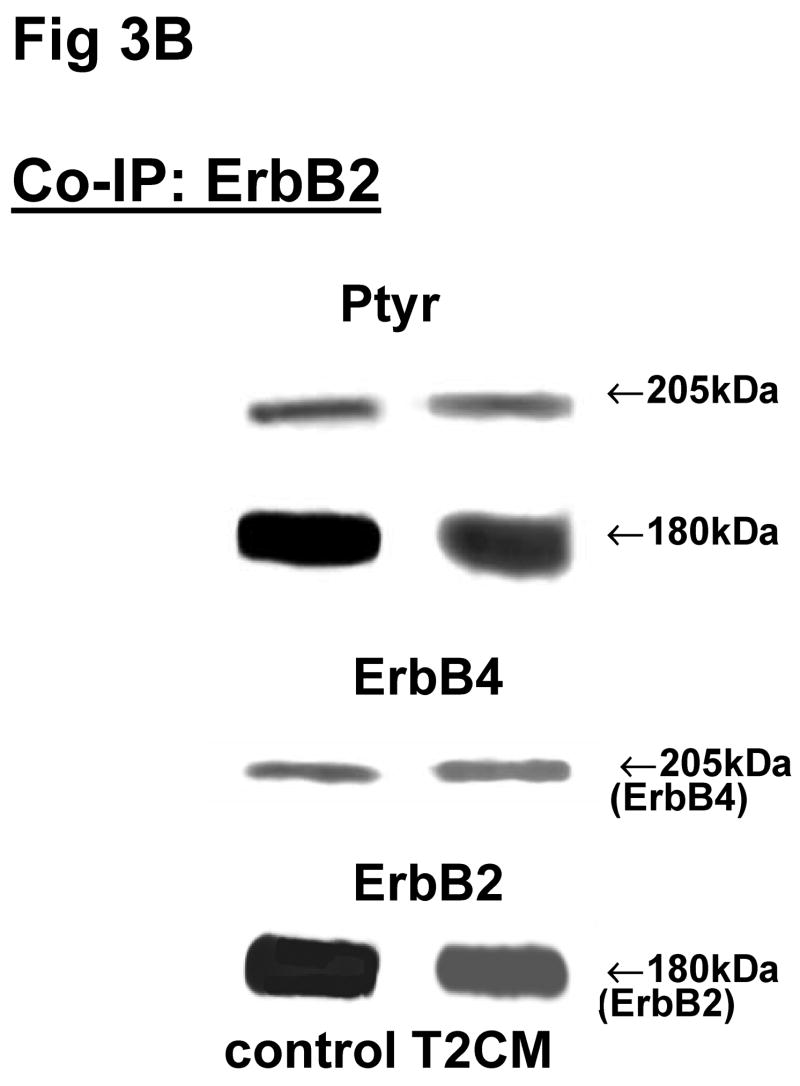
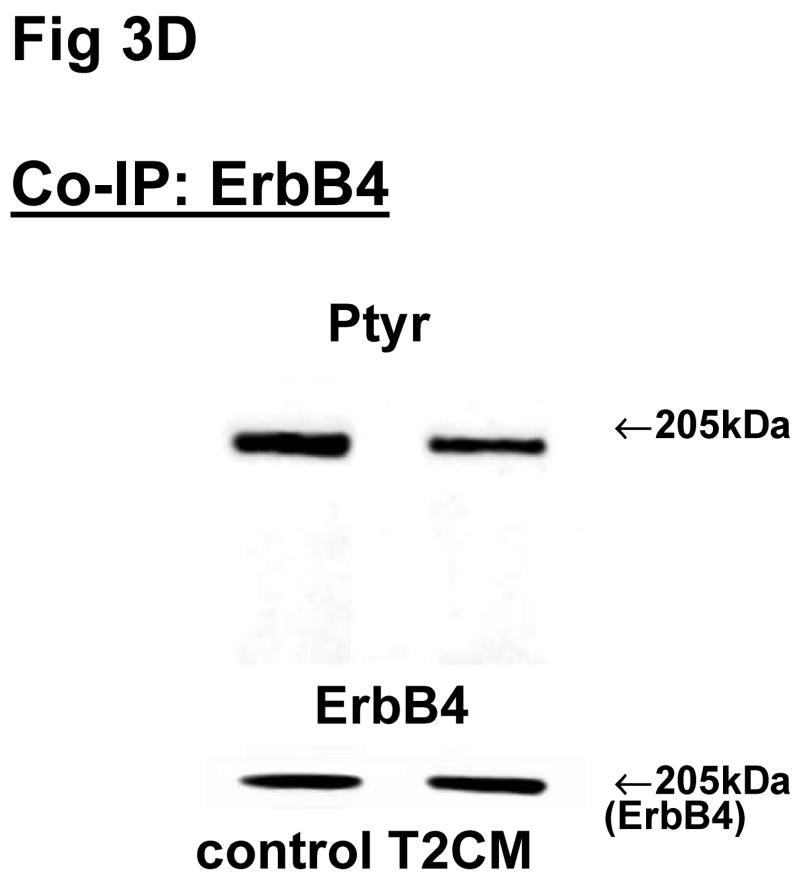
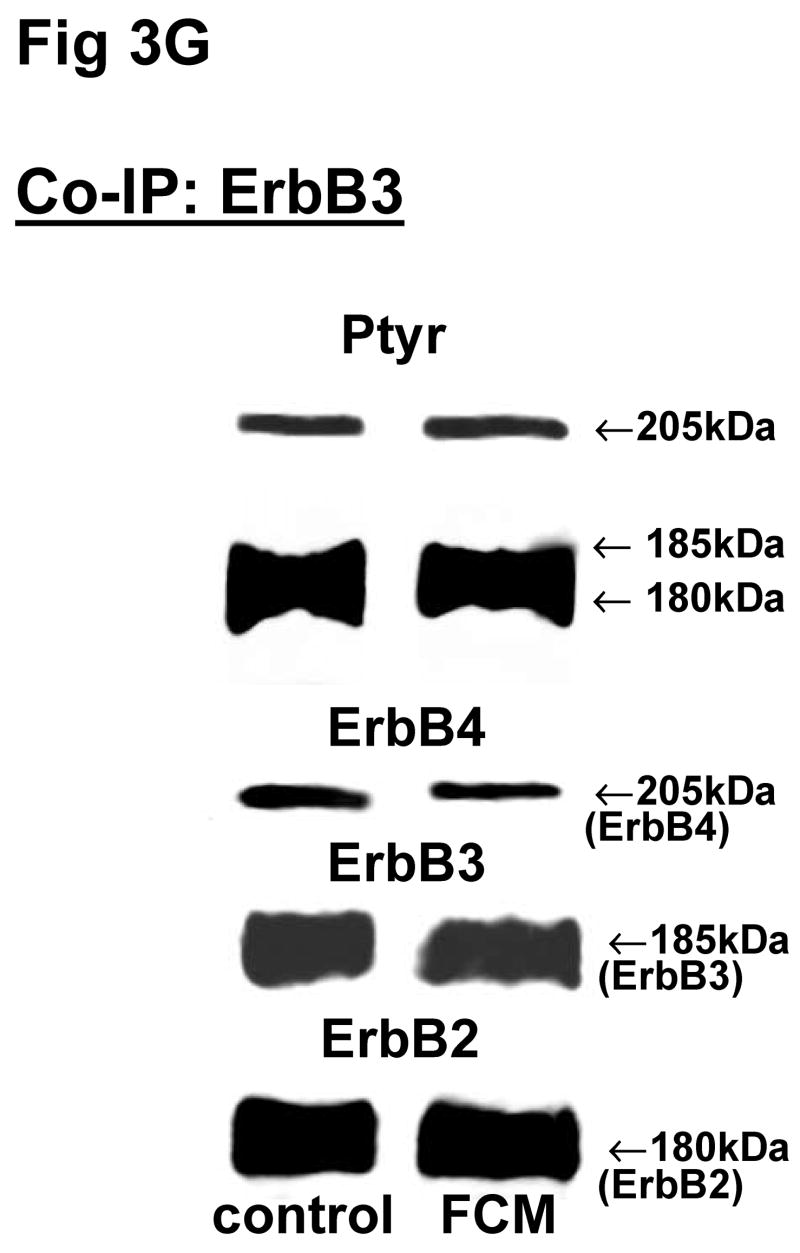
The above Co-IP experiments demonstrated the reproducible appearance of specific heterodimer partners with changes in receptor phosphorylation in response to ligand stimulation. In order to provide more insight into the role of ErbB receptor heterodimerization and phosphorylation in fibroblast-epithelial type II cell communication in fetal lung development, we studied the ErbB receptor heterodimerization and phosphorylation in E19 fetal rat fibroblasts and type II cells at baseline and after treatment with conditioned medium from the opposite cell type. T2CM treatment of fibroblasts, and FCM treatment of type II cells for 24 hours produced the same dimer patterns as that with growth factor stimulation (Fig 3). Table 4 summarizes the densitometry analysis of the phosphorylated proportion of total protein and amount of total receptor protein of both precipitated and co-precipitated ErbB receptor dimers in response to the conditioned media. In fibroblasts with T2CM treatment, the ErbB1 protein amounts were significantly decreased to 77±7% (N=5, P<0.05), compared to the untreated cells. In the contrary FCM stimulated type II cell ErbB1 receptor protein amount to 167±12% (N=4, P<0.05) of untreated cells when immunoprecipitated with an antibody to ErbB1 and to 204±10% (N=3, P<0.05) of untreated cells when immunoprecipitated with an antibody to ErbB4. These data suggest that the ErbB1/ErbB4 dimers play an important role in the mesenchyme-epithelial communication in E19 fetal rats. The conditioned media treatments did not change the dimer patterns in the fibroblasts and type II cells.
Fig 3. Co-immunoprecipitation of ErbB receptor heterodimers in fibroblast-epithelial communication in rat fetal lung.

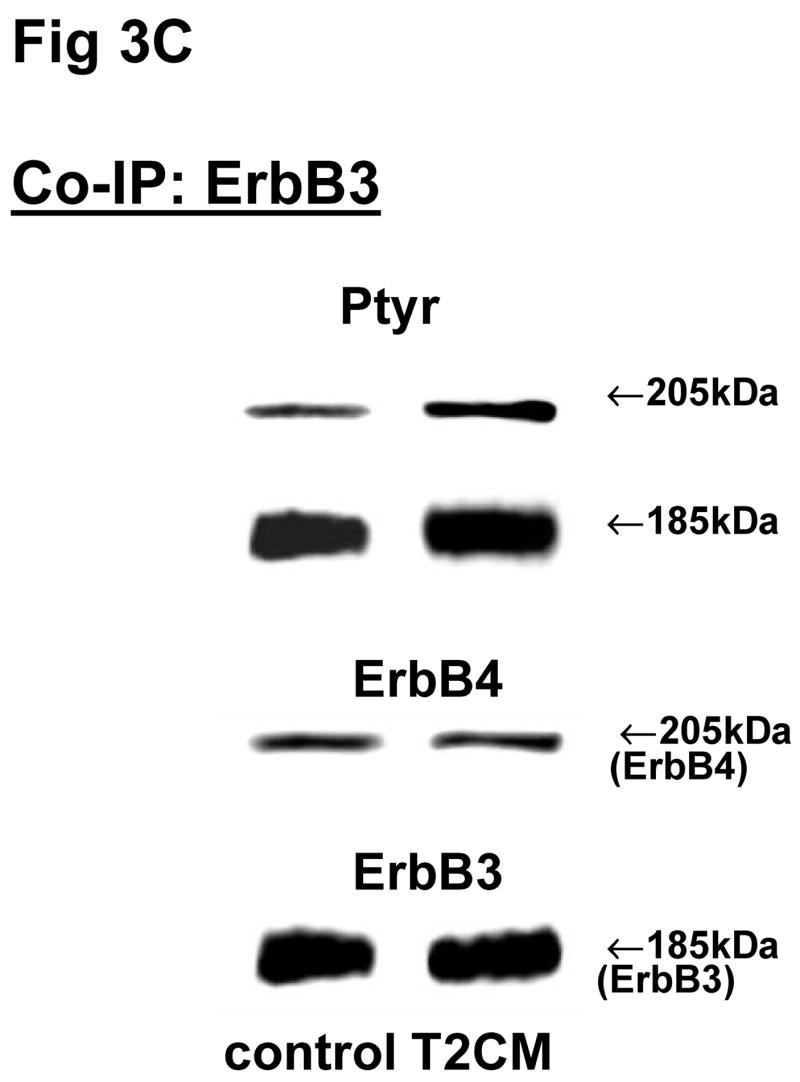

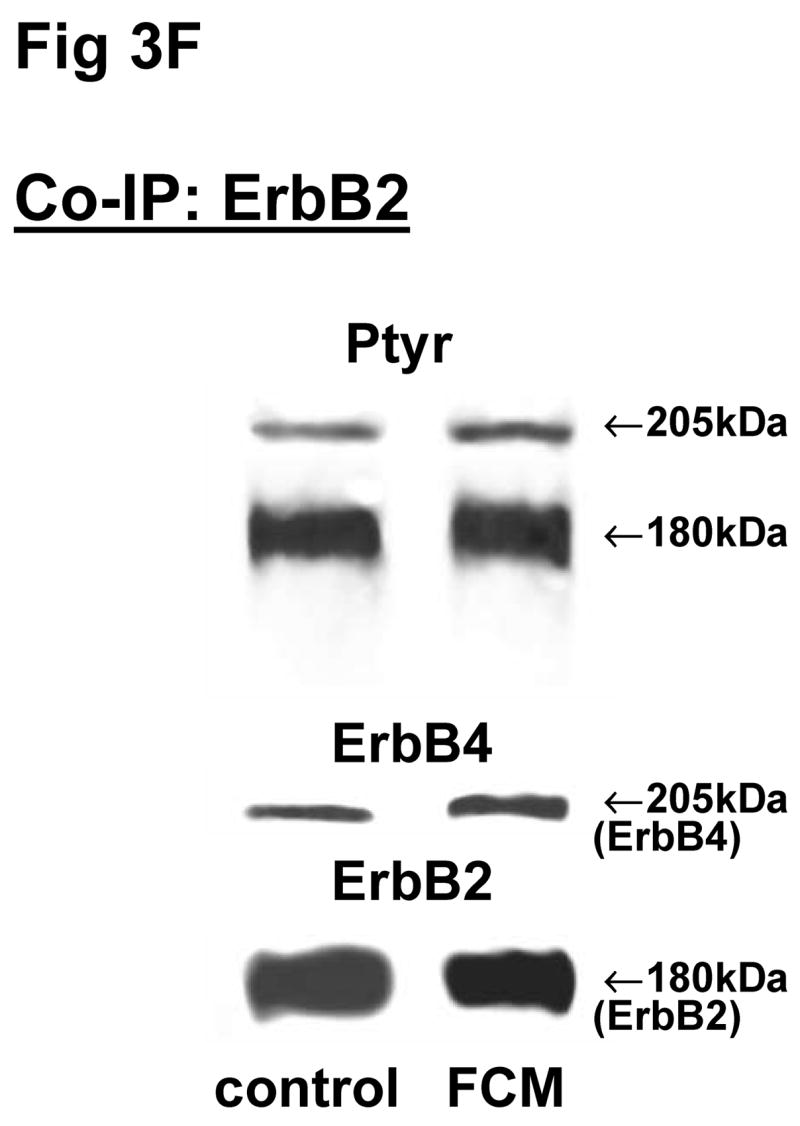

Cells were treated with the conditioned medium from opposite cell types and immunoprecipitated with antibody to ErbB1 (A, E), ErbB2 (B, F), ErbB3 (C, G), ErbB4 (D, H) under co-immunoprecipitation conditions. Panels show representative blots for fibroblasts (A, B, C, D) and type II cells (E, F, G, H); illustrated results were consistently found in 3–5 independent experiments.
Table 4. The phosphorylation and protein amount of ErbB receptors and the heterodimers in fetal rat fibroblasts and type II cells in response to a 24 hours treatment with conditioned medium from opposite cell type.
Receptor protein values are percentage of treatment signals over control. Phosphorylation values represent proportion of phosphorylated signal over total receptor protein normalized to the experimental control.
| Dimers | ErbB1 | ErbB2 | ErbB3 | ErbB4 | ||||
|---|---|---|---|---|---|---|---|---|
| IP | Ptyr | protein | Ptyr | protein | Ptyr | protein | Ptyr | protein |
| Fibroblasts | ||||||||
| ErbB1 | 170±36 | 77±7# | 118±27 | 87±13 | ||||
| ErbB2 | 137±35 | 65±21# | 112±57 | 106±12 | ||||
| ErbB3 | 96±14 | 126±26 | 171±74 | 114±19 | ||||
| ErbB4 | 80±18 | 112±41 | ||||||
| Type II cells | ||||||||
| ErbB1 | 86±10 | 167±12# | 292±130 | 111±11 | 126±25 | 107±8 | ||
| ErbB2 | 86±2 | 116±12 | 76±3* | 139±10 | ||||
| ErbB3 | 119±16 | 102±14 | 119±16 | 99±7 | 127±44 | 106±15 | ||
| ErbB4 | 81±19 | 204±10# | 110±41 | 139±10 | ||||
Data are Mean±SEM, N=3–5;
P<0.05 compared to unstimulated control with Dunnett multiple comparison test (to correct for using controls for 4 treatment corrections).
P<0.05 compared to unstimulated control with paired t test. Ptyr: Phosphotyrosine.
Down regulation of ErbB4 in fibroblasts affects surfactant lipid synthesis in type II cells
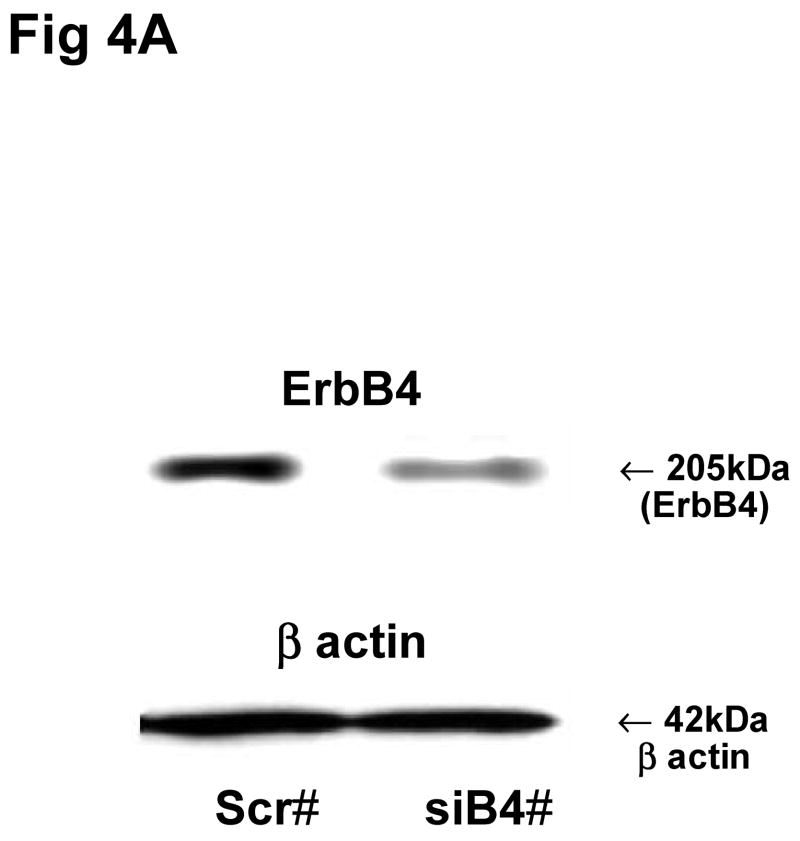
ErbB4 was a common dimer partner for each of the other ErbB receptors, and the studies with conditioned media suggested that the ErbB1/ErbB4 dimer was an important ErbB heterodimer in mesenchyme-epithelial communication. Therefore we further investigated the importance of ErbB4 in mesenchyme-epithelial communication by gene silencing of ErbB4 with small interfering RNA (siRNA) in fibroblasts. The ErbB4 receptor protein amount in fibroblasts was down regulated to 77±5% by transfection with ErbB4 siRNAs compared to scrambled siRNA treatment (Fig 4A, 4B). During the final 24 hours of ErbB4 down regulation FCM was collected from the fibroblasts and used to treat type II cells. The synthesis of surfactant phospholipid was measured by assay of incorporated 3H choline into DSPC. Treatment of the type II cells with FCM from the fibroblasts with ErbB4 siRNA treatment increased choline incorporation into DSPC by 43±13% over the treatment with FCM from scrambled siRNA (Fig 4C). These data imply that the inhibition of ErbB4 in the fibroblasts may promote the release of factors that stimulate the synthesis of surfactant phospholipids in type II cell.
Fig 4. ErbB4 down regulation in fibroblasts and the effect of FCM from the ErbB4 down regulated fibroblasts on DSPC synthesis in type II cells.
A: Representative blots of total ErbB4 receptor proteins (upper panel) and β actin (lower panel) in female fibroblasts. #Scr: scrambled siRNA, siB4: ErbB4 siRNAs. B: Densitometric analysis of ErbB4 receptor protein amounts in fibroblasts. Values (Mean±SEM, N=8) represent the ratio of receptor protein to β actin and then normalized by scrambled siRNA treatments. *: P<0.05. C: 3H Choline incorporation into DSPC in type II cells. Values represent ratio of DPM to protein amounts and then normalized by scrambled siRNA treatments. Mean±SEM, N=24. **P<0.01.
Localization of ErbB receptors in fetal rat lung type II cells
Confocal fluorescence microscopy was used to investigate the effects of NRG1β and FCM on cellular localization and co-localization of the most common dimerization partner, ErbB4, in the epithelial type II cells. We focused these studies on type II cells because of their importance for fetal lung surfactant synthesis and also because of the broad impact of ErbB ligands and FCM on ErbB4 receptor phosphorylation in type II cells. In untreated type II cells, ErbB4 was diffusely localized in the nuclear region and showed a less intense signal in the cytoplasm (Fig 4A, 4B, 4C, green fluorescent color, left panels). ErbB1 and ErbB2 were also mainly localized in the nuclei (Fig 4A, 4B, red fluorescent color), whereas ErbB3 appeared as discrete clumps in the nuclei suggestive of nucleolar localization (Fig 4C, red fluorescent color). In the absence of treatment there was some evidence of co-localization of ErbB4 and ErbB2, but little visible evidence for co-localization with the other receptors. Exposure to NRG1β for 24 hours led to a relocation of all ErbB receptors out of the nucleus into the cytoplasm (Fig 4A, 4B, 4C, middle panels). Exposure to FCM did not change the cellular localization of ErbB4, but induced relocalization of ErbB1 and ErbB3 out of the nucleus (Fig 4A, 4C, red fluorescent color, right panels). ErbB2 localization was also not dramatically changed by FCM compared to the untreated cells (Fig 4B, red fluorescent color, right panel), intensifying the co-localization of both receptors.
Discussion
ErbB receptor heterodimerization and phosphorylation in late developing lungs
In this study, we found that the four ErbB receptors are present in both fibroblasts and alveolar epithelial type II cells of late gestational fetal rat lung. Their expression patterns and responses to ligand stimulation are cell- and ligand-specific, with a stronger expression of ErbB1, ErbB2, and ErbB3 in alveolar epithelial type II cells. ErbB receptor function is recognized to have a significant role in the development and propagation of several types of cancer, including lung cancers [42]. Studies have focused on the heterodimerization dynamics of ErbB receptors using both tumor cell lines and cell lines engineered to express only specific ErbB receptors [41]. Such studies have identified ErbB2 as the preferred heterodimerization partner for each of the other three ErbB receptors [5,13]. In contrast, in this study we found in the developing rat lung that ErbB4 is the most common heterodimerization partner. There are important differences between tumor cell lines, engineered cell lines, and primary fetal cells that would lead one to expect differences in results. Our findings correlate with functional results in other systems. For example in the reproductive system, ErbB1 and ErbB4 must act in concert in the development of adult reproductive function for rat female sexual differentiation [30]. Numerous other studies have reported that heterodimerization of ErbB4 with other ErbB receptors forms a high affinity binding complex which enhances the level of auto-phosphorylation and results in a significant modification of the ligand-induced biological response [10,31,38]. There is a general impression that ErbB4 expression is found within more differentiated compartments. Given its widespread expression in embryonic as well as adult tissue a regulatory function in multiple systems would not be surprising [3]. ErbB2, which has no known ligand, must dimerize with a ligand-activated ErbB receptor to signal its effect [17,39]. It is possible that in fetal type II cells NRG1β, which is secreted by lung fibroblasts and found in the FCM [8], signals some effects on surfactant synthesis through ErbB2/ErbB4 dimers such as we identified in this study.
ErbB4 was consistently co-immunoprecipitated with other ErbB receptors and was clearly visible by Western blotting in both rat fetal lung fibroblasts and type II cells, however Western blot did not show the presence of any other ErbB receptors in fibroblasts after co-immunoprecipitation with ErbB4 antibody. We can postulate two possible reasons for this observation, both related to the possible stoichiometry of heterodimer formation. One possibility is that when immunoprecipitating with antibody for another receptor, e.g. ErbB1, the vast majority of ErbB1 heterodimers were with ErbB4, so ErbB4 could readily be detected on Western blot, whereas when immunoprecipitating with antibody for ErbB4, its heterodimers were split among ErbB1, ErbB2 and ErbB3 such that the relative proportions of these co-precipitants was too low to be detected on Western blot. The second possible reason for the lack of ErbB4 co-precipitants in fibroblasts may be the three-dimensional structural change of the dimers after antibody binding [7,11]. Different antibodies may bind at different sites of the complex, changing the conformation and affect the dimer stability, leading to inconsistent results in both directions. However the stoichiometry of the ligands and receptors in the heterodimer complexes are unknown. Further studies on the structural level of ErbB heterodimerization will be needed to fully resolve this issue.
EGF and TGFα are ligands for ErbB1, and NRG1β is a ligand for both ErbB3 and ErbB4. These ligands can activate their homo- or heterodimers [9,41]. It has been suggested that the ErbB heterodimers may form secondary heterotetramers [15]. The formation of secondary hetero-oligomers can be induced by a ligand for a third ErbB protein. Therefore EGF and TGFα may stimulate the formation of 2/4 or 4/4 heterodimers and phosphorylation via ErbB1. The third ErbB protein might not be precipitated because of the small amount and weak binding. Actually the heterodimerization of ErbB receptors and the mechanism of phosphorylation have been proven more complicated or diverse by interactions with some cell surface receptors outside the ErbB family [36] and intracellular adaptors and scaffolding systems [6,14,16].
ErbB receptor cellular localization in late developing lungs
In our fluorescent confocal microscope studies in type II cells all four ErbB receptors exhibited strong staining in the nuclei and re-localized to the extra-nuclear cytoplasmic region after NRG1β treatment. This effect was most prominent for ErbB4. Since we studied the effect of a 24-hour exposure to NRG we cannot compare the confocal localization data to the short-term (2 minutes) stimulatory effect of NRG on receptor dimerization or phosphorylation seen in co-immunoprecipitation. Compared to NRG1β, FCM induced a differential effect on ErbB2 and ErbB4, in which co-localization of these two receptors was observed by confocal imaging. Surprisingly, even though ErbB4 precipitated with all other ErbB receptors in fetal type II cells after FCM exposure, there was only co-localization found for ErbB2 and ErbB4 (Fig 5B). Interestingly, 24-hour exposure to NRG led to a decrease in phosphorylation of the co-localized ErbB4.
Fig 5. Cellular localization of ErbB receptors in fetal rat alveolar epithelial type II cells.

Confocal immunofluorescent microscopy was used to identify the cellular localization of the major dimerization partner ErbB4 (green), with ErbB1 (red) (A), ErbB2 (red) (B), or ErbB3 (red) (C) under control conditions (left panels) and after treatment with either NRG (middle panels) or FCM (right panels) for 24 hours.
We did not observe polarization (apical versus basal) characteristics, which likely is due to the fact that cells do not develop polarization when cultured on plastic. Studies of ErbB1 immunostaining in sections of fetal lung show no evidence of polarization of ErbB1 [34]. Both this feature and the lack of a distinct membrane concentration of ErbB receptors has been observed under some conditions in other cell types by ourselves [46] and by others [23,44]. A feature common to these observations is ongoing receptor activation (i.e. phosphorylation) in culture. Thus, the confocal image result may reflect the dynamic nature of the ErbB receptors in our experiments.
With NRG1β treatments, the majority of internalized receptor is in the cytoplasm, indicating specific signal responses to NRG1β. Others have shown that stimulation with ligands can cause direct trafficking from membrane to cytoplasm and nucleus [23,19,27]. The nuclear function of the ErbB receptors is just beginning to be understood. Our results are all consistent with a role of ErbB receptors in the cell-cell communication process controlling fetal lung maturation.
ErbB receptor regulation in mesenchyme – epithelia communication in late developing lungs
Fetal lung maturation requires communication between the mesenchyme and adjacent type II cells, which is mediated via direct contact or soluble factors [40]. Conditioned medium from type II cells significantly decreased ErbB1 and ErbB2 protein content in fibroblasts, whereas FCM significantly stimulated ErbB1 and ErbB4 protein content in type II epithelial cells. At this time in lung development the surfactant stimulatory effect of lung fibroblast secreted factors is just starting, requiring glucocorticoid exposure to fully stimulate it. Structurally, there is the development of mesenchymal condensation, such that a specific subset of fibroblasts becomes closely associated with the developing acinar structure and the type II cells [2]. This subset of fibroblasts takes on the phenotype of lipofibroblasts, which actively regulate type II cell maturation via production of maturational factors. We speculate that T2CM at this time contains factors which promote this remodeling and the development of the lipofibroblast phenotype [37,43].
Loss of balance of ErbB receptors may lead to functional changes. Down regulation of ErbB4 by ErbB4 siRNA in E19 rat type II cells impairs surfactant synthesis [45]. Deletion of pulmonary ErbB4 expression in cardiac rescued ErbB4 transgenic mice also impairs surfactant synthesis in late fetal development (unpublished data). In this study, down regulation of ErbB4 in fibroblasts caused increased type II cell surfactant DSPC synthesis, suggesting that fibroblast-type II cell communication promoting surfactant synthesis is negatively regulated in fibroblasts by ErbB4 activity. ErbB1/ErbB4 dimers may play an important regulatory role in late fetal lung development. Dimerization and activation of ErbB receptors are normally triggered by binding with their ligands. The heterodimerization and phosphorylation observed in untreated fibroblasts and type II cells may be induced by autocrine mechanisms. This high endogenous activity may make the primary pulmonary cells less sensitive to exogenous growth factor stimulation than may be observed in some cell lines (unpublished data).
We conclude that ErbB receptors are differentially expressed in the fetal lung and that ErbB4 heterodimers are particularly important in late gestation fetal lung development. The role of ErbB4 heterodimers may be to distinctly control maturational processes in both fibroblasts and type II cells to specifically regulate the development of surfactant synthesis. Further understanding of how ErbB receptors act in developing lung should provide important insight into how the development of surfactant synthesis is regulated.
Acknowledgments
This work was funded by National Institute of Health HL37930 (Nielsen), HL04436 (Dammann), Da 378/3-1 (Dammann), the Peabody Foundation and the Charles Hood Foundation, Boston, MA USA. Confocal microscopy studies were performed at the Image Center for Neuroscience Research at Tufts University. We thank Rob Jackson PhD and Lai Ding PhD of the Image Center (P30 NS047243 (Jackson)) and Anne Kane M.D. of the GRASP Center (GRASP P30 DK34928) for technical assistance; and Sujatha M. Ramadurai MD, MaryAnn V. Volpe MD, and Lucia D. Pham of the Division of Newborn Medicine for consulting support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alroy I, Yarden Y. The ErbB signaling network in embryogenesis and oncogenesis: signal diversification through combinatorial ligand-receptor interactions. FEBS Lett. 1997;410:83. doi: 10.1016/s0014-5793(97)00412-2. [DOI] [PubMed] [Google Scholar]
- 2.Caniggia I, Tseu I, Han RN, Smith BT, Tanswell K, Post M. Spatial and temporal differences in fibroblast behavior in fetal rat lung. Am J Physiol. 1991;261:L424–L433. doi: 10.1152/ajplung.1991.261.6.L424. [DOI] [PubMed] [Google Scholar]
- 3.Carpenter G. ErbB-4: mechanism of action and biology. Exp Cell Res. 2003;284:66. doi: 10.1016/s0014-4827(02)00100-3. [DOI] [PubMed] [Google Scholar]
- 4.Carraway KL, III, Burden SJ. Neuregulins and their receptors. Curr Opin Neurobiol. 1995;5:606. doi: 10.1016/0959-4388(95)80065-4. [DOI] [PubMed] [Google Scholar]
- 5.Carraway KL, III, Rossi EA, Komatsu M, Price-Schiavi SA, Huang D, Guy PM, Carvajal ME, Fregien N, Carraway CA, Carraway KL. An intramembrane modulator of the ErbB2 receptor tyrosine kinase that potentiates neuregulin signaling. J Biol Chem. 1999;274:5263. doi: 10.1074/jbc.274.9.5263. [DOI] [PubMed] [Google Scholar]
- 6.Chattopadhyay A, Vecchi M, Ji Q, Mernaugh R, Carpenter G. The role of individual SH2 domains in mediating association of phospholipase C-gamma1 with the activated EGF receptor. J Biol Chem. 1999;274:26091. doi: 10.1074/jbc.274.37.26091. [DOI] [PubMed] [Google Scholar]
- 7.Cho HS, Leahy DJ. Structure of the extracellular region of HER3 reveals an interdomain tether. Science. 2002;297:1330. doi: 10.1126/science.1074611. [DOI] [PubMed] [Google Scholar]
- 8.Dammann CE, Nielsen HC, Carraway KL., III Role of neuregulin-1 beta in the developing lung. Am J Respir Crit Care Med. 2003;167:1711. doi: 10.1164/rccm.200205-468OC. [DOI] [PubMed] [Google Scholar]
- 9.Ebner R, Derynck R. Epidermal growth factor and transforming growth factor-alpha: differential intracellular routing and processing of ligand-receptor complexes. Cell Regul. 1991;2:599. doi: 10.1091/mbc.2.8.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fitzpatrick VD, Pisacane PI, Vandlen RL, Sliwkowski MX. Formation of a high affinity heregulin binding site using the soluble extracellular domains of ErbB2 with ErbB3 or ErbB4. FEBS Lett. 1998;431:102. doi: 10.1016/s0014-5793(98)00737-6. [DOI] [PubMed] [Google Scholar]
- 11.Garrett TP, McKern NM, Lou M, Elleman TC, Adams TE, Lovrecz GO, Zhu HJ, Walker F, Frenkel MJ, Hoyne PA, Jorissen RN, Nice EC, Burgess AW, Ward CW. Crystal structure of a truncated epidermal growth factor receptor extracellular domain bound to transforming growth factor alpha. Cell. 2002;110:763. doi: 10.1016/s0092-8674(02)00940-6. [DOI] [PubMed] [Google Scholar]
- 12.Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, Lemke G. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378:390. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- 13.Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997;16:1647. doi: 10.1093/emboj/16.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashimoto Y, Katayama H, Kiyokawa E, Ota S, Kurata T, Gotoh N, Otsuka N, Shibata M, Matsuda M. Phosphorylation of CrkII adaptor protein at tyrosine 221 by epidermal growth factor receptor. J Biol Chem. 1998;273:17186. doi: 10.1074/jbc.273.27.17186. [DOI] [PubMed] [Google Scholar]
- 15.Huang GC, Ouyang X, Epstein RJ. Proxy activation of protein ErbB2 by heterologous ligands implies a heterotetrameric mode of receptor tyrosine kinase interaction. Biochem J. 1998;331( Pt 1):113. doi: 10.1042/bj3310113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji QS, Chattopadhyay A, Vecchi M, Carpenter G. Physiological requirement for both SH2 domains for phospholipase C-gamma1 function and interaction with platelet-derived growth factor receptors. Mol Cell Biol. 1999;19:4961. doi: 10.1128/mcb.19.7.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klapper LN, Glathe S, Vaisman N, Hynes NE, Andrews GC, Sela M, Yarden Y. The ErbB-2/HER2 oncoprotein of human carcinomas may function solely as a shared coreceptor for multiple stroma-derived growth factors. Proc Natl Acad Sci U S A. 1999;96:4995. doi: 10.1073/pnas.96.9.4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee KF, Simon H, Chen H, Bates B, Hung MC, Hauser C. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature. 1995;378:394. doi: 10.1038/378394a0. [DOI] [PubMed] [Google Scholar]
- 19.Lin SY, Makino K, Xia W, Matin A, Wen Y, Kwong KY, Bourguignon L, Hung MC. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol. 2001;3:802. doi: 10.1038/ncb0901-802. [DOI] [PubMed] [Google Scholar]
- 20.LOWRY OH, ROSEBROUGH NJ, FARR AL, RANDALL RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265. [PubMed] [Google Scholar]
- 21.Miettinen PJ, Berger JE, Meneses J, Phung Y, Pedersen RA, Werb Z, Derynck R. Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature. 1995;376:337. doi: 10.1038/376337a0. [DOI] [PubMed] [Google Scholar]
- 22.Murray S, Pham L, Dammann CEL, Nielsen HC. Inhibition of ErbB Receptor Function in Murine and Human Pulmonary Epithelial Cells. 2004 [Google Scholar]
- 23.Ni CY, Murphy MP, Golde TE, Carpenter G. gamma -Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001;294:2179. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen HC. Epidermal growth factor influences the developmental clock regulating maturation of the fetal lung fibroblast. Biochim Biophys Acta. 1989;1012:201. doi: 10.1016/0167-4889(89)90097-9. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen HC, Martin A, Volpe MV, Hatzis D, Vosatka RJ. Growth factor control of growth and epithelial differentiation in embryonic lungs. Biochem Mol Med. 1997;60:38. doi: 10.1006/bmme.1996.2560. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen HC, Torday JS. Anatomy of fetal rabbit gonads and the sexing of fetal rabbits. Lab Anim. 1983;17:148. doi: 10.1258/002367783780959411. [DOI] [PubMed] [Google Scholar]
- 27.Offterdinger M, Schofer C, Weipoltshammer K, Grunt TW. c-erbB-3: a nuclear protein in mammary epithelial cells. J Cell Biol. 2002;157:929. doi: 10.1083/jcb.200109033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olski TM, Noegel AA, Korenbaum E. Parvin, a 42 kDa focal adhesion protein, related to the alpha-actinin superfamily. J Cell Sci. 2001;114:525. doi: 10.1242/jcs.114.3.525. [DOI] [PubMed] [Google Scholar]
- 29.Post M, Smith BT. Histochemical and immunocytochemical identification of alveolar type II epithelial cells isolated from fetal rat lung. Am Rev Respir Dis. 1988;137:525. doi: 10.1164/ajrccm/137.3.525. [DOI] [PubMed] [Google Scholar]
- 30.Prevot V, Lomniczi A, Corfas G, Ojeda SR. erbB-1 and erbB-4 receptors act in concert to facilitate female sexual development and mature reproductive function. Endocrinology. 2005;146:1465. doi: 10.1210/en.2004-1146. [DOI] [PubMed] [Google Scholar]
- 31.Riese DJ, Komurasaki T, Plowman GD, Stern DF. Activation of ErbB4 by the bifunctional epidermal growth factor family hormone epiregulin is regulated by ErbB2. J Biol Chem. 1998;273:11288. doi: 10.1074/jbc.273.18.11288. [DOI] [PubMed] [Google Scholar]
- 32.Riese DJ, Stern DF. Specificity within the EGF family/ErbB receptor family signaling network. Bioessays. 1998;20:41. doi: 10.1002/(SICI)1521-1878(199801)20:1<41::AID-BIES7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 33.Riethmacher D, Sonnenberg-Riethmacher E, Brinkmann V, Yamaai T, Lewin GR, Birchmeier C. Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nature. 1997;389:725. doi: 10.1038/39593. [DOI] [PubMed] [Google Scholar]
- 34.Rosenblum DA, Volpe MV, Dammann CE, Lo YS, Thompson JF, Nielsen HC. Expression and activity of epidermal growth factor receptor in late fetal rat lung is cell- and sex-specific. Exp Cell Res. 1998;239:69. doi: 10.1006/excr.1997.3888. [DOI] [PubMed] [Google Scholar]
- 35.Ruocco S, Lallemand A, Tournier JM, Gaillard D. Expression and localization of epidermal growth factor, transforming growth factor-alpha, and localization of their common receptor in fetal human lung development. Pediatr Res. 1996;39:448. doi: 10.1203/00006450-199603000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Saito Y, Haendeler J, Hojo Y, Yamamoto K, Berk BC. Receptor heterodimerization: essential mechanism for platelet-derived growth factor-induced epidermal growth factor receptor transactivation. Mol Cell Biol. 2001;21:6387. doi: 10.1128/MCB.21.19.6387-6394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schultz CJ, Torres E, Londos C, Torday JS. Role of adipocyte differentiation-related protein in surfactant phospholipid synthesis by type II cells. Am J Physiol Lung Cell Mol Physiol. 2002;283:L288–L296. doi: 10.1152/ajplung.00204.2001. [DOI] [PubMed] [Google Scholar]
- 38.Shelly M, Pinkas-Kramarski R, Guarino BC, Waterman H, Wang LM, Lyass L, Alimandi M, Kuo A, Bacus SS, Pierce JH, Andrews GC, Yarden Y. Epiregulin is a potent pan-ErbB ligand that preferentially activates heterodimeric receptor complexes. J Biol Chem. 1998;273:10496. doi: 10.1074/jbc.273.17.10496. [DOI] [PubMed] [Google Scholar]
- 39.Sliwkowski MX, Schaefer G, Akita RW, Lofgren JA, Fitzpatrick VD, Nuijens A, Fendly BM, Cerione RA, Vandlen RL, Carraway KL., III Coexpression of erbB2 and erbB3 proteins reconstitutes a high affinity receptor for heregulin. J Biol Chem. 1994;269:14661. [PubMed] [Google Scholar]
- 40.Smith BT. Lung maturation in the fetal rat: acceleration by injection of fibroblast-pneumonocyte factor. Science. 1979;204:1094. doi: 10.1126/science.582216. [DOI] [PubMed] [Google Scholar]
- 41.Sweeney C, Fambrough D, Huard C, Diamonti AJ, Lander ES, Cantley LC, Carraway KL., III Growth factor-specific signaling pathway stimulation and gene expression mediated by ErbB receptors. J Biol Chem. 2001;276:22685. doi: 10.1074/jbc.M100602200. [DOI] [PubMed] [Google Scholar]
- 42.Sweeney C, Miller JK, Shattuck DL, Carraway KL., III ErbB receptor negative regulatory mechanisms: implications in cancer. J Mammary Gland Biol Neoplasia. 2006;11:89. doi: 10.1007/s10911-006-9015-3. [DOI] [PubMed] [Google Scholar]
- 43.Torday JS, Torres E, Rehan VK. The role of fibroblast transdifferentiation in lung epithelial cell proliferation, differentiation, and repair in vitro. Pediatr Pathol Mol Med. 2003;22:189. doi: 10.1080/pdp.22.3.189.207. [DOI] [PubMed] [Google Scholar]
- 44.Williams CC, Allison JG, Vidal GA, Burow ME, Beckman BS, Marrero L, Jones FE. The ERBB4/HER4 receptor tyrosine kinase regulates gene expression by functioning as a STAT5A nuclear chaperone. J Cell Biol. 2004;167:469. doi: 10.1083/jcb.200403155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zscheppang K, Liu W, Volpe MV, Nielsen HC, Dammann CEL. ErbB4 Regulates Fetal Surfactant Synthesis. Proc Am Thor Soc. 2006;3:A672. [Google Scholar]
- 46.Zscheppang K, Korenbaum E, Bueter W, Ramadurai SM, Nielsen HC, Dammann CE. ErbB receptor dimerization, localization, and co-localization in mouse lung type II epithelial cells. Pediatr Pulmonol. 2006;41:1205. doi: 10.1002/ppul.20518. [DOI] [PubMed] [Google Scholar]