Summary
Epsin and AP180 are essential components of the endocytotic machinery which controls internalization of protein receptors and other macromolecules at the cell surface. Epsin and AP180 are recruited to the plasma membrane by their structurally and functionally related N-terminal ENTH and ANTH domains that specifically recognize PtdIns(4,5)P2. Here, we show that membrane anchoring of the ENTH and ANTH domains is regulated by the acidic environment. Lowering the pH enhances PtdIns(4,5)P2 affinity of the ENTH and ANTH domains reinforcing their association with lipid vesicles and monolayers. The pH dependency is due to the conserved histidine residues of the ENTH and ANTH domains, protonation of which is necessary for the strong PtdIns(4,5)P2 recognition, as revealed by liposome binding, surface plasmon resonance, NMR, monolayer surface tension and mutagenesis experiments. The pH sensitivity of the ENTH and ANTH domains is reminiscent to the pH dependency of the FYVE domain suggesting a common regulatory mechanism of membrane anchoring by a subset of the PI-binding domains.
Keywords: PtdIns(4,5)P2; ENTH; epsin; ANTH; membrane
Introduction
Clathrin-mediated endocytosis is one of the major mechanisms for internalization of cell surface receptors, growth factors and other extracellular macromolecules inside the cell.1-3 Clathrin promotes invagination of the plasma membrane and formation of a coated pit which then buds into the cytoplasm and after fission produces a small transport vesicle containing the internalized cargo. Once endocytosed, the vesicle fuses with early endosomes and the cargo either recycles back to the plasma membrane or is forwarded to the lysosome for degradation. The initial steps of endocytosis, membrane budding and formation of vesicles require the clathrin triskeleton assembly stimulated by the adaptor complex AP-2 and multiple accessory co-factors.1,4,5 Two accessory proteins, Epsin and AP180, initiate the formation of a pit by docking to the plasma membrane and recruiting and promoting polymerization of clathrin. It has been reported that membrane association of Epsin and AP180 is primarily mediated by their amino-terminal domains that recognize PtdIns(4,5)P2.4,6-11 Once bound to the lipid, Epsin modifies the membrane curvature and induces tubulation of liposome bilayers and the plasma membrane in living cells,8 while AP180 stimulates clathrin assembly at newly invaginated synaptic vesicles but does not deform membranes.6,9
The Epsin N-terminal homology (ENTH) domain, a conserved ∼150-residue module, was discovered in 199912,13 and has since been identified in all members of the Epsin family (SMART). Found exclusively at the amino-termini, the ENTH domain recruits Epsin to the plasma membrane through specific, ∼0.37 μM-affinity interaction with PtdIns(4,5)P2.7 Cell localization studies indicate that PtdIns(4,5)P2 recognition by the ENTH domain is sufficient for the strong membrane association. The PI binding of the ENTH domain also plays a role in membrane anchoring of clathrin, AP-2, Eps15 and intersectin as these components of endocytotic machinery bind a long unstructured sequence following the ENTH domain in the majority of Epsin proteins.12,14-16 Mutations in the ENTH domain that abolish lipid binding or truncation of the entire domain have a dominant negative effect on endocytosis of epidermal growth factor, likely due to the loss of the ability to recruit the endocytotic assembly to the plasma membrane.7,8,10
The ENTH domain folds in a compact structure consisting of eight α-helices packed in three twisted helical hairpins7,8,17. While in the ligand-free state of the domain the N-terminal 14-residue sequence is unstructured, it becomes ordered forming an amphipathic α-helix (helix 0 or α0) upon binding to PtdIns(4,5)P2 or Ins(1,4,5)P3, a head group of the lipid.7,8 Binding of the ENTH domain to PtdIns(4,5)P2 facilitates deformation of the membrane due to the insertion of α0 into the membrane interior, pushing the headgroups apart and reducing the energy barrier of bending.8,9 The ENTH-like AP180 N-terminal homology (ANTH) domain is nearly twice as large, consisting of 280 amino acids that form ten α-helices, however, it bears a similar topology to the ENTH domain.6,18 In fact, the ANTH domain structure includes N-terminal α-helices of the ENTH domain followed by three additional α-helices.
Despite the structural and functional similarity, the ENTH and ANTH domains bind PtdIns(4,5)P2 in a different manner. The Arg7, Arg8 and Lys11 of α0, Arg25 of α1, Arg63 of α3, Lys69, and His73 of α4 in the ENTH domain form a deep basic pocket that accommodates the negatively charged headgroup of PtdIns(4,5)P2.8 In contrast, the ANTH domain coordinates PtdIns(4,5)P2 via the solvent exposed Lys28 of α1, and Lys38, Lys39, Lys40 and His41 of α2.6 These surface residues are highly conserved in the ANTH domain distinguishing it from the ENTH domain, in which this region is neither conserved nor is it involved in the binding to PtdIns(4,5)P2.
In this work we demonstrate that anchoring of the ENTH and ANTH domains to PtdIns(4,5)P2-enriched membranes is pH dependent and is enhanced by the acidic environment. The pH sensitivity is due to the conserved histidine residues located in the PtdIns(4,5)P2 binding pockets and involved in the inositol headgroup coordination. Our results, derived from in vitro experiments using monolayer penetration, nuclear magnetic resonance (NMR), surface plasmon resonance and liposome binding assays, combined with the mutagenesis data and in vivo localization of fluorescently-tagged proteins, provide new insights into the multivalent mechanism of membrane docking by the ENTH and ANTH domain-containing proteins. The pH dependent recognition of PtdIns(4,5)P2 by the ENTH and ANTH domains is reminiscent to the pH-dependent binding of the FYVE domain to PtdIns(3)P-enriched bilayers suggesting a common regulatory mechanism of membrane anchoring by a subset of PI-binding domains.
Results and Discussion
Targeting of the ENTH and ANTH domains to PtdIns(4,5)P2-containing bilayers is pH-dependent
To understand the role of physiological pH alterations on the ability of the ENTH and ANTH domains to associate with PtdIns(4,5)P2-containing membranes, the protein-lipid interactions in a range of pHs were investigated by liposome binding assays. The GST-fusion ENTH and ANTH domains were incubated with small unilamellar vesicles (SUVs) composed of lipids normally found in plasma membranes including phosphatidylcholine (PC), phosphatidylethanolamine (PE) and PtdIns(4,5)P2 at pH 6.0, 7.0 or 8.0. Following the centrifugation, the partitioning of proteins between supernatant and pelleted fraction was examined. As demonstrated in Figure 1 a and b, one third of the ENTH domain and a half of the ANTH domain retained in the pelleted liposome fraction at a low pH of 6.0. At each progressively higher pH, both domains were increasingly redistributed to the supernatant. The densitometry analysis of the gel bands revealed that binding to PtdIns(4,5)P2-enriched vesicles was reduced from 36 to 19% for the ENTH domain and from 63 to 46% for the ANTH domain as a result of alkalization of the buffer from pH 6.0 to 8.0 (Fig. 1, d and e). The GST tag alone was found in the soluble fraction in all buffers tested indicating that it is the ENTH and ANTH domains that bind PtdIns(4,5)P2-containing liposomes in a pH-dependent manner (Fig. 1 c). In the absence of PtdIns(4,5)P2, neither domain associated with SUVs, confirming that PtdIns(4,5)P2 is required for the membrane localization of these proteins.
Figure 1.
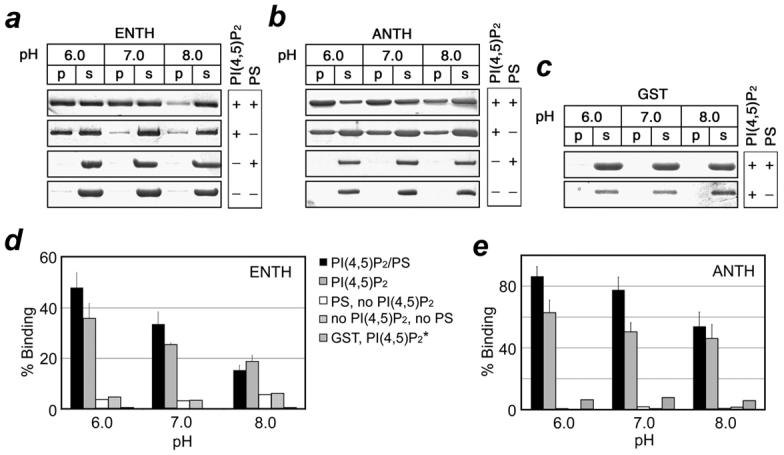
pH-dependent binding of the Epsin ENTH and AP180 ANTH domains to PtdIns(4,5)P2-enriched liposomes. The SDS/PAGE gels (a-c) and histograms (d, e) show partitioning of the ENTH and ANTH domains between supernatant (s) and the liposome pellet (p) at indicated pHs. *Association of GST with PC/PE/PtdIns(4,5)P2 vesicles in the absence (d) and presence (e) of PS. The experimental points were averaged over at least three measurements.
Affinities of the ENTH and ANTH domains for PtdIns(4,5)P2-containing vesicles increase in the acidic environment
To quantitatively assess the effect of pH, kinetic and equilibrium binding constants of the ENTH and ANTH domains’ association with bilayers were measured by surface plasmon resonance (SPR) (Fig. 2 and Table 1). We first tested binding of the Epsin ENTH domain to POPC/POPE/PtdIns(4,5)P2 (78:20:2) vesicles at different pHs using POPC/POPE (80:20) vesicles as a control. It has previously been shown that in the absence of PtdIns(4,5)P2 both domains are unable to bind liposomes even at high micromolar concentrations of the proteins.9 In the presence of PtdIns(4,5)P2, the ENTH domain localized to the vesicles, however the binding affinity was significantly decreased when pH of the buffer was raised from 6.0 to 8.0. Thus the ENTH domain exhibited a 9.8 nM affinity for POPC/POPE/PtdIns(4,5)P2 vesicles at pH 6.0, however this interaction was 8-fold weaker at pH 7.4 and 34-fold weaker at pH 8.0 (Table 1). Concurrently a decrease of ka and an increase of kd were observed, most likely due to the reduced electrostatic interactions and a lesser degree of membrane penetration by the ENTH domain.19,20
Figure 2.
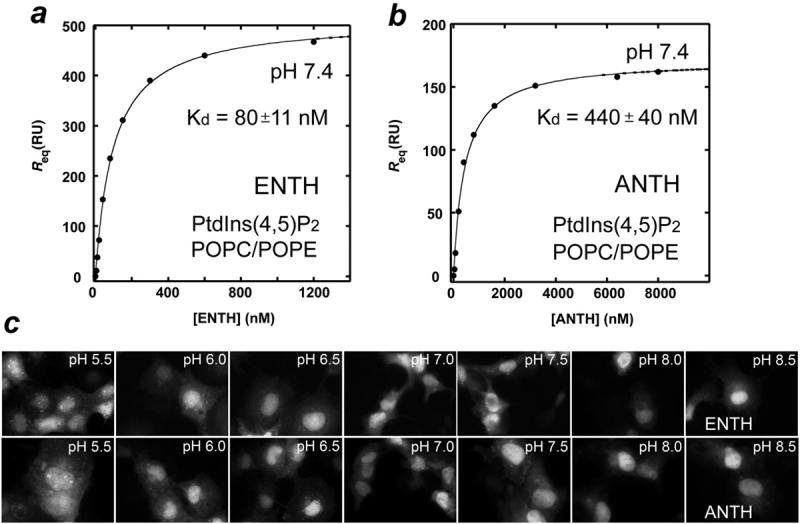
Affinities of the ENTH and ANTH domains for PtdIns(4,5)P2-containing membranes increase in the acidic environment. a and b, SPR measurements used to determine Kds of the ENTH and ANTH domains association with POPC/POPE/PtdIns(4,5)P2 vesicles. The solid line represents a theoretical curve obtained based on Rmax and Kd values determined by nonlinear least squares analysis. c, Changes in localization of EGFP-ENTH and EGFP-ANTH domains in COS-1 cells upon varying the cytosolic pH. Cultures were incubated in solutions buffered to the indicated pH values for 15 min. The cells were visualized by fluorescence microscopy.
Table 1.
Binding properties of the Epsin ENTH and AP180 ANTH domains and Epsin H73A mutant.
| Protein | ka M-1 s-1 × 105 | kd s-1 × 10-2 | Kd M × 10-9 | Fold Changea |
|---|---|---|---|---|
| POPC/POPE/PtdIns(4,5)P2 (78:20:2) | ||||
| ENTH pH 6.0 | (8.2 ± 0.6) | (0.80 ± 0.07) | (9.8 ± 1.0) | - |
| H73A ENTH pH 6.0 | (5.8 ± 0.4) | (2.8 ± 0.3) | (48 ± 6) | 5 |
| ENTH pH 7.4 | (4.4 ± 0.5) | (3.5 ± 0.3) | (80 ± 11) | 8 |
| H73A ENTH pH 7.4 | (2.4 ± 0.3) | (5.9 ± 0.4) | (250 ± 36) | 26 |
| ENTH pH 8.0 | (1.9 ± 0.2) | (6.2 ± 0.4) | (330 ± 60) | 34 |
| H73A ENTH pH 8.0 | (1.0 ± 0.2) | (9.3 ± 0.5) | (930 ± 190) | 95 |
| ANTH pH 6.0 | ---------- | ---------- | (95 ± 12) | - |
| ANTH pH 7.4 | ---------- | ---------- | (440 ± 40) | 5 |
| ANTH pH 8.0 | ---------- | ---------- | (1200 ± 120) | 13 |
| POPC/POPE/POPS/PtdIns(4,5)P2 (63:20:15:2) | ||||
| ENTH pH 6.0 | (9.7 ± 0.6) | (0.25 ± 0.03) | (2.6 ± 0.3) | - |
| H73A ENTH pH 6.0 | (8.0 ± 0.5) | (0.81 ± 0.04) | (10 ± 0.8) | 4 |
| ENTH pH 7.4 | (9.2 ± 0.4) | (1.8 ± 0.2) | (20 ± 2.4) | 8 |
| H73A ENTH pH 7.4 | (6.8 ± 0.6) | (3.6 ± 0.3) | (53 ± 6) | 20 |
| ENTH pH 8.0 | (4.5 ± 0.5) | (3.1 ± 0.3) | (69 ± 10) | 26 |
| H73A ENTH pH 8.0 | (3.5 ± 0.4) | (5.8 ± 0.5) | (170 ± 24) | 65 |
| ANTH pH 6.0 | ---------- | ---------- | (18 ± 4) | - |
| ANTH pH 7.4 | ---------- | ---------- | (98 ± 12) | 5 |
| ANTH pH 8.0 | ---------- | ---------- | (290 ± 40) | 16 |
Decrease of the affinity relative to the Kd of wild type ENTH at pH 6.0
A similar enhancement of the membrane binding affinity upon acidification was seen for the AP180 ANTH domain. We monitored the ANTH domain association with PtdIns(4,5)P2-containing vesicles at pH 6.0, 7.4, and 8.0. Since kd of this interaction at pH 8.0 was too fast to accurately measure, the affinity was assessed by equilibrium binding analysis (Table 1). As expected the ANTH domain displayed a significant increase in affinity in acidic conditions and a decrease in basic. The Kd value of the ANTH domain association with POPC/POPE/PtdIns(4,5)P2 vesicles was found to be 95 nM at pH 6.0. This binding was reduced by 5-fold and 13-fold at pH 7.4 and 8.0, respectively (Table 1). Thus our data demonstrate that pH mediates docking of the Epsin ENTH and AP180 ANTH domains to PtdIns(4,5)P2-containing bilayers.
The cytosolic pH can regulate subcellular localization of the Epsin ENTH and AP180 ANTH domains
To examine the role of intracellular pH for in vivo localization of the proteins, enhanced green fluorescent protein (EGFP)-fusion Epsin ENTH and AP180 ANTH domains were expressed in COS-1 cells that were briefly incubated in media buffered to various pH values (Fig. 2 c). Mammalian cells maintain an intracellular pH of approximately 7.3,21 however the neutral nature of the cytosol can be altered by changing the pH of the medium.22 We investigated the subcellular distribution of EGFP-ENTH and EGFP-ANTH at pH 5.5, 6.0, 6.5, 7.0, 7.5, 8.0 and 8.5 by fluorescence microscopy. In media buffered to pH 5.5-6.5, both EGFP-ENTH and EGFP-ANTH were found in membrane microdomains and the nucleus (Fig. 2 c).17,23 When pH of the media was gradually increased, the EGFP signal of the microdomain-associated ENTH and ANTH domains was diminished and an increase in the nuclear EGFP signal was observed. In basic conditions only a nuclear pool of EGFP-ENTH and EGFP-ANTH was detected. The most significant changes in the ENTH and ANTH domains’ localization occurred in the physiological pH range of 6.5 to 7.5. Thus the Epsin ENTH domain and AP180 ANTH domain localize to the membrane microdomains and the nucleus at low cytosolic pH, whereas at high pH values the proteins are found primarily in the nucleus.
PtdIns(4,5)P2 binding of the ENTH and ANTH domains is pH-sensitive
To test whether the pH-dependence is attributed to the direct interaction of the ENTH or ANTH domain with PtdIns(4,5)P2, the protein-lipid binding was characterized in the absence of membrane-mimetics by PIP2-pull down assays and NMR spectroscopy. The GST-fusion ENTH and ANTH domains were incubated with PtdIns(4,5)P2 immobilized beads at pH 6.0, 7.0 and 8.0 and association with the lipid was examined by sedimentation and SDS-PAGE and quantified by densitometry (Fig. 3, a and b). Both ENTH and ANTH domains showed a steady decrease in binding to PIP2-beads when pH was raised from 6.0 to 8.0 indicating that the PtdIns(4,5)P2 interaction of these modules is indeed pH sensitive and is enhanced by the acidic media.
Figure 3.
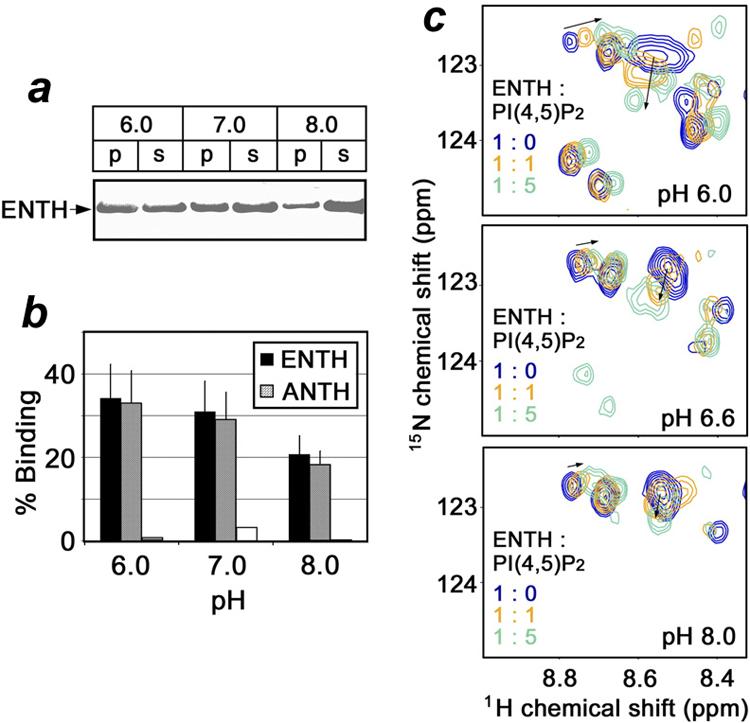
The direct interaction of the ENTH and ANTH domains with PtdIns(4,5)P2 is pH-sensitive and is enhanced by lowering the pH. The SDS/PAGE gel (a) and histogram (b) display the distribution of the ENTH and ANTH domains between supernatant (s) and pelleted PIP2-beads (p) at indicated pHs. (c) Superimposed 1H-15N HSQC spectra of the 15N-labeled ENTH domain (0.2 mM) recorded during addition of C4-PtdIns(4,5)P2 at pH 6.0, 6.6 and 8.0. The relative concentrations of the ENTH domain and the lipid [PI(4,5)P2] are color coded.
The enhancement of PtdIns(4,5)P2 binding in a low pH environment was substantiated by resonance perturbations seen in NMR spectra of the Epsin ENTH domain. We collected 1H-15N heteronuclear single quantum coherence (HSQC) spectra of the uniformly 15N-labeled protein at pH 6.0, 6.6 and 8.0 as soluble C4-PtdIns(4,5)P2 lipid was gradually added. As demonstrated in Figure 3 c, progressive changes in the amide resonances of the ENTH domain were observed at pH 6.0, however at pH 8.0 an excess of PtdIns(4,5)P2 induced barely any chemical shift perturbations, implying that the binding affinity is low under basic conditions. The fast exchange on the NMR time-scale also suggested that the ENTH domain binds water-soluble short chain C4-PtdIns(4,5)P2 weaker than the long chain lipid embedded in the vesicles (Table 1 and Fig. 3, c). Taken together our data indicate that the direct interaction of the ENTH and ANTH domains with PtdIns(4,5)P2 lipid is pH dependent and becomes stronger in the acidic conditions.
Conserved His73 residue of the ENTH domain mediates PtdIns(4,5)P2 binding
Our previous studies reveal that PtdIns(3)P binding of the FYVE domain is modulated by the protonation state of His residues.24 The PtdIns(4,5)P2 binding pocket of the ENTH domain contains a conserved His73 residue which forms a hydrogen bond to the 4-phosphate group of PtdIns(4,5)P2 as seen in the crystal structure of the Epsin ENTH domain (Fig. 4 and Fig. 5).8 To determine whether His73 plays a role in the pH dependent membrane anchoring it was replaced with Ala and binding of the H73A mutant protein to POPC/POPE/PtdIns(4,5)P2 vesicles was probed at different pH values. We found that the H73A mutant associates with vesicles 5-fold weaker than the wild type ENTH domain at pH 6.0, 3-fold weaker at pH 7.4 and 2.8-fold weaker at pH 8.0 (Table 1). Overall, when compared to the affinity of the mutant measured at pH 6.0, the binding affinity of H73A was decreased by 5-fold at pH 7.4 and 20-fold at pH 8.0. In contrast, the wild type ENTH domain showed an 8- and 34-fold decrease in the affinity at pH 7.4 and 8.0, respectively. Thus our data indicate that His73 is necessary for the strong membrane anchoring and its protonation increases the binding affinity.
Figure 4.
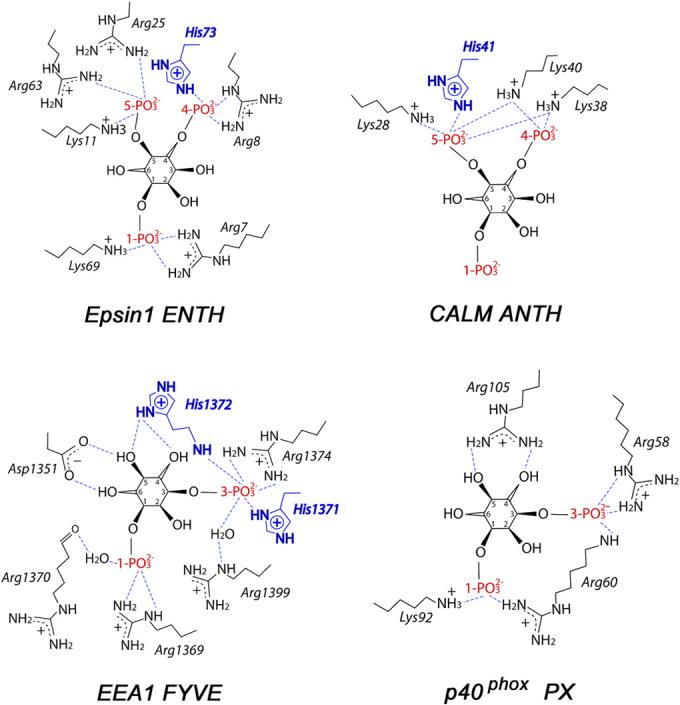
Schematic diagrams showing PtdIns(4,5)P2 headgroup coordination by Epsin ENTH and CALM ANTH domains, and PtdIns(3)P headgroup coordination by EEA1 FYVE and p40phox PX domains (PDB codes 1HOA, 1HFA, 1JOC and 1H6H). Only charged residues of the proteins are depicted for clarity.
Figure 5.
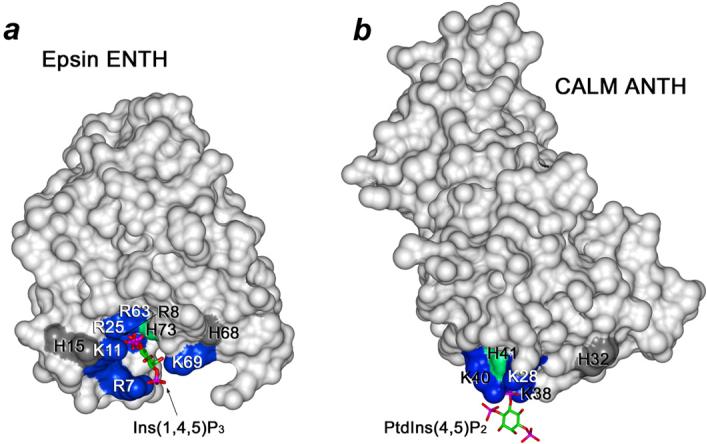
PtdIns(4,5)P2 binding pocket defined from the crystal structure of the Epsin ENTH domain complexed with Ins(1,4,5)P3 (PDB 1HOA) (a) and the crystal structure of the CALM ANTH domain in complex with PtdIns(4,5)P2 (PDB 1HFA) (b). The basic binding site residues are labeled and colored blue. The conserved His74 of the Epsin ENTH domain and His41 of the ANTH domain are in green. Other surface His residues are colored gray.
The fact that the H73A mutant did not entirely abolish the interaction indicates that the binding site lysine and arginine residues play a key role in coordinating the lipid (Fig. 4 and Fig. 5). Furthermore, mutation of His73 decreases the pH sensitivity of binding but does not eliminate it, suggesting that other His residues may be responsible for the remaining pH dependence. Thus, the surface residues His15 and His68 of the ENTH domain and His32 of the ANTH domain are located in close proximity to the PtdIns(4,5)P2 binding site and may contribute to the interactions with acidic membranes (Fig. 5). It may also indicate the existence of another concomitantly occurring process, i.e. deprotonation of PtdIns(4,5)P2 in basic conditions. The pKa values of the 4- and 5-phosphate groups are estimated to be 6.7 and 7.6,25 therefore in the physiological pH range the net charge of PtdIns(4,5)P2 undergoes a substantial change from -3 (pH<6.0) to -5 (pH>8.0). The basic environment promotes deprotonation of both phosphate groups resulting in complete ionization of this phospholipid and accumulation of two extra negative charges. On the contrary to deprotonation of His73, this should strengthen PtdIns(4,5)P2 coordination by the positively charged lysine and arginine residues of the ENTH domain.
Nonspecific electrostatic interactions with PS enhance the ENTH and ANTH domain binding to PtdIns(4,5)P2-containing vesicles
The inner leaflet of the plasma membrane contains over 10% of anionic phospholipids such as PS and PI.26 To determine whether nonspecific electrostatic interactions with acidic lipids other than PtdIns(4,5)P2 contribute to the pH dependent behavior of the ENTH and ANTH domains, liposome binding assays were carried out using PC/PE/PS/PtdIns(4,5)P2 vesicles. As shown in Figure 1 d and e, both domains bound PS-containing vesicles better than the neutral vesicles at all pH values tested. In the presence of PS, the fraction of the liposome-bound ENTH and ANTH domains increased from 36 to 50% and from 63 to 90%, respectively, suggesting that nonspecific electrostatic interactions with PS amplify the binding affinity. The pH sensitivity of the ENTH and ANTH domains toward PS-containing vesicles was clearly preserved (Fig. 1, d and e).
To further assess the effect of PS, we measured kinetic and thermodynamic parameters of the ENTH and ANTH binding to POPC/POPE/POPS/PtdIns(4,5)P2 (63:20:15:2) vesicles (Table 1). The ENTH domain exhibited an increase in affinity at all pH levels when PS was present. At pH 6.0 the protein was bound to the PS-enriched vesicles with a nearly 4-fold higher affinity than to the vesicles without PS, demonstrating the significance of nonspecific electrostatic interactions for the membrane targeting. Furthermore, the ENTH domain displayed the same 4-fold increase in the affinity at pH 7.4 and 8.0, indicating similar contributions of non-specific electrostatic interactions. This was further supported by examining the binding of H73A to POPC/POPE/POPS/PtdIns(4,5)P2 vesicles at different pHs. The H73A mutant showed a ∼5-fold higher affinity for vesicles containing PS than those without this lipid at any pH. A comparable 4- to 5-fold increase of the affinity was observed for the ANTH domain association with POPC/POPE/POPS/PtdIns(4,5)P2 vesicles across the pH range tested. This again demonstrates that nonspecific electrostatic interactions are essential for membrane recruitment of the AP180 ANTH domain.
Membrane insertion of the ENTH domain is promoted by acidic pH
Recent reports have demonstrated that the ENTH domain penetrates PtdIns(4,5)P2-enriched membranes by inserting helix 0 into the bilayer.8,9 In contrast, the ANTH domain lacking exposed hydrophobic residues near the PtdIns(4,5)P2 binding interface does not penetrate and interacts with membranes through a bridging model (Fig. 5). To examine the effect of the pH on the membrane insertion we tested both proteins by monolayer penetration experiments. Phospholipid monolayers at the air-water interface serve as a highly sensitive tool for measuring the membrane penetrating ability of peripheral proteins.9,20,27,28 POPC/POPE (80:20) or POPC/POPE/PtdIns(4,5)P2 monolayers of a given initial surface pressure (π) were spread at a constant area and the change in surface pressure after the injection of proteins was monitored. Δπ is inversely proportional to π and an extrapolation of Δπ versus π yields the critical surface pressure πχ, which specifies an upper limit of π that a protein can penetrate into.29 Because the surface pressure of cell membranes and large unilamellar vesicles is estimated to be in a range of 30-35 dyne/cm,20,28 for a protein to penetrate these bilayers its πχ value should be above 30 dyne/cm.
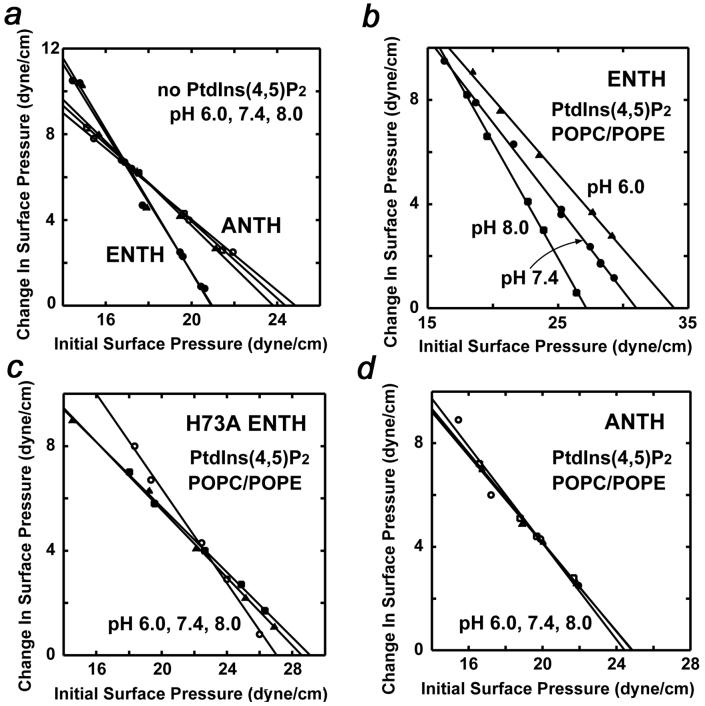
Figure 6 a shows that both Epsin ENTH and AP180 ANTH domains have low intrinsic penetrating ability toward a POPC/POPE monolayer and the πχ values (21 and 25 dyne/cm) remain essentially unchanged when the experiments were performed at pH 6.0, 7.4 or 8.0. Thus in the absence of PtdIns(4,5)P2 the ENTH and ANTH domains do not penetrate POPC/POPE membranes nor does the pH influence the insertion. We next examined the penetration of the Epsin ENTH domain into PtdIns(4,5)P2 containing monolayers at different pH levels (Fig. 6 b). When 3 mol% PtdIns(4,5)P2 was incorporated in the monolayer, the ENTH domain penetration increased to 27 dyne/cm at pH 8.0. Furthermore, the πχ value rose to 31 and 34.5 dyne/cm at pH 7.4 and 6.0, respectively, indicating that the acidic environment enhances not only the membrane affinity but also the penetrating capability of the ENTH domain. Based on the estimated πχ values, the ENTH domain should not significantly penetrate biological membranes at pH above 7.4. Unlike the wild type protein, H73A mutant lost the pH dependency (Fig. 6 c). In fact, it had much lower penetrating power than the wild type ENTH at pH 6.0 and 7.4, however similarly to the wild type protein, H73A was unable to effectively insert into the monolayer at pH 8.0. These data confirm the critical role of His73 in PtdIns(4,5)P2 binding and further support the notion that the membrane penetration of the ENTH domain requires efficient PtdIns(4,5)P2 binding which in turn is induced by the acidic media.
Figure 6.
Monolayer penetration of Epsin ENTH and AP180 ANTH domains. a, Insertion of wild type ENTH (filled symbols) and ANTH (open symbols) into a POPC/POPE (80:20) monolayer at pH 6.0 (triangle), 7.4 (circle) and 8.0 (square) monitored as a function of π0. Penetration of wild type ENTH (b) and H73A ENTH (c) into a POPC/POPE/PtdIns(4,5)P2 (77:20:3) monolayer at pH 6.0 (▲), 7.4 (●) and 8.0 (■). d, Penetration of AP180 ANTH into a POPC/POPE/PtdIns(4,5)P2 (77:20:3) monolayer at pH 6.0 (Δ), 7.4 ( ) and 8.0 (
) and 8.0 ( ).
).
Our recent studies show that the AP180 ANTH domain binds PtdIns(4,5)P2 containing vesicles primarily through electrostatic interactions and doesn’t penetrate the bilayers.27 To rule out the possibility that pH influences the penetration of the ANTH domain, we monitored its binding to POPC/POPE/PtdIns(4,5)P2 monolayers at different pH values (Fig. 6 d). Although the ANTH domain bound PtdIns(4,5)P2 containing membranes much stronger at pH 6.0, we did not observe an increase in monolayer penetration. Likewise, no significant penetration into PtdIns(4,5)P2 containing monolayers was seen at pH 7.4 or 8.0. This and the data described above corroborate that PtdIns(4,5)P2 recognition of the ANTH domain is pH dependent but the higher affinity in acidic conditions is not caused by changes in the membrane penetration of this module.
Conclusions
Our results reveal a pH-dependent mechanism of membrane docking by the Epsin ENTH and AP180 ANTH domains. At the core of this mechanism is the ability of His residues involved in PtdIns(4,5)P2 coordination to undergo a rapid protonation/deprotonation transition in the physiological pH range, and thus to modulate the strength of the lipid binding (Fig. 4 and Fig. 5). The acidic environment causes protonation of essential His73 and His41 residues of the ENTH and ANTH domains, which in turn facilitates binding of these proteins to PtdIns(4,5)P2. Alignment of the ENTH and ANTH domain sequences shows conservation of both His residues (Fig. 7), suggesting that pH-dependent membrane association may be a general characteristic of these modules.
Figure 7.
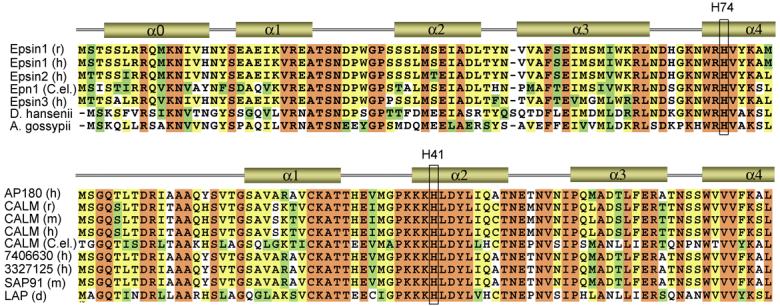
Alignment of the ENTH and ANTH domain sequences: absolutely, moderately and weakly conserved residues are colored brown, yellow and green, respectively. The secondary structure is shown above the sequences (only N-terminal part of the domains is depicted). The conserved His73 residue of the ENTH domain and His41 of the ANTH domain are labeled.
The pH dependent recognition of PtdIns(4,5)P2 by the ENTH and ANTH domains resembles the pH sensitivity of the FYVE finger.24 Because of a single His residue, however, they display much weaker pH dependence than the FYVE domain, which contains two adjacent His residues in the PtdIns(3)P binding site and exhibits sharp pH dependency (Fig. 4). The pH sensitive function of the ENTH, ANTH and FYVE domains suggests a common anchoring mechanism for a subset of PI-binding modules, distinguishing them from the PX domain, which contains no histidine residues in the PI-binding pocket and is shown to be pH independent.24
The pH-dependence can influence the functions of Epsin and AP180 in cells with unusual cytosolic pH levels and in normal cells during physiological processes that involve changing of pH. Intracellular pH often fluctuates in response to cell growth, development, and apoptosis, with pH varying from 6.3 to 7.8.30-33 Even wider fluctuations are detected in anomalous cells during ischemia,34 inflammation35 and cancer36. We found that EGFP-Epsin ENTH and AP180 ANTH association with membrane microdomains is diminished when the intracellular pH is increased. This suggests that subcellular localization of the ENTH and ANTH domains can vary due to fluctuations in acidification or alkalization of the cytosol. Future studies are required to establish the significance of the pH sensing by the ENTH and ANTH domains and to determine whether the membrane localization of these proteins is altered in biological processes that are accompanied by the changes in cytosolic pH.
Methods
Subcloning, expression and purification of proteins
Human AP180 ANTH domain (residues 1 - 289) and rat Epsin ENTH domain (residues 1-164 and 1-144) were expressed in E. coli BL21 (DE3) pLysS strain in LB or in 15NH4Cl supplemented minimal media (Isotec). Bacteria were harvested by centrifugation after induction with IPTG (0.1 mM) and lysed using a French press (18,000 psi, 4°C). The unlabeled or 15N-uniformly labeled GST-fusion proteins were purified on a glutathione sepharose 4B column (Amersham). The GST tag was cleaved with Thrombin (Sigma). The proteins were concentrated using Millipore concentrators (Millipore) and purified by FPLC. The buffer was exchanged into 20 mM Tris or perdeuterated (d)11-Tris, 150 mM KCl, 1 mM d10-dithiothreitol, 50 μM 4-amidinophenylmethane sulfonyl fluoride and 7% 2H2O. The purity of the proteins was determined by SDS-PAGE and 1H NMR.
PCR Mutagenesis
Site-directed mutagenesis of Epsin ENTH domain was performed using a QuikChange kit (Stratagene). The sequence of the H73A ENTH construct was confirmed by DNA sequencing.
Liposome binding
The liposome binding assays were performed as described in.24 Briefly, solutions of PC, PE, PS (Avanti) and C16-PtdIns(4,5)P2 (Echelon Biosciences Inc.) dissolved in CHCl3:MeOH:H2O (65:25:4) were mixed and dried down under vacuum. The lipids were resuspended in 50 mM Tris, 100 mM KCl pH 7.0 and incubated at 64°C for 1 hour. The liposomes were then frozen in liquid nitrogen and thawed at 37°C for three cycles. The liposome solution was passed through an Avanti extruder to produce 1.0 μm liposomes. Liposomes were collected by centrifugation at 25,000 × g for 10 minutes and resuspended to a final concentration of 2 mM total lipids in 100 μl 20 mM Tris, 100 mM KCl buffer, pH 6.0, 7.0 or 8.0. Liposomes were incubated with the GST-fusion ENTH and ANTH domains or GST (2-5 μg/ml final protein concentration) for 30 min at room temperature and then collected again by centrifugation. The liposome pellets were resuspended in 100 μl of buffer and analyzed by SDS-PAGE and Coomassie brilliant blue staining.
PIP2-Beads pull-down
The 2-5 μg ENTH domain, ANTH domain or GST were incubated with 100 μl of PIP2 beads (Echelon Biosciences Inc.) suspended in bis-Tris or Tris pH 6.0, 7.0 or 8.0 buffers at room temperature for 1 hr. The beads fraction and supernatant were separated by centrifugation and analyzed by SDS-PAGE.
NMR spectroscopy and lipid titrations
NMR spectra were recorded at 25°C on a Varian INOVA 500 MHz spectrometer. The 1H-15N heteronuclear single quantum coherence (HSQC) spectra of 0.2 mM uniformly 15N-labeled ENTH domain were collected using 1024 t1 increments of 2048 data points, 96 number of increments and spectral widths of 7500 and 1367 Hz in the 1H and 15N dimensions, respectively. PtdIns(4,5)P2 binding was characterized at pH 6.0, 6.6 and 8.0 by monitoring chemical shift changes in 1H-15N HSQC spectra of the ENTH domain as soluble C4-PtdIns(4,5)P2 was added stepwise up to 1 mM.
Monolayer measurements
Insertion of the ENTH and ANTH domains into a monolayer was investigated by measuring a change in the surface pressure (π) of invariable surface area during addition of the proteins. The experiments were performed using a 1 ml circular Teflon trough and wire probe connected to a Kibron MicroTrough X (Kibron, Inc., Helsinki). A lipid monolayer containing various combinations of phospholipids was spread onto the subphase composed of either 10 mM KH2PO4 and 0.16 M KCl at pH 6.0 or 10 mM HEPES and 0.16 M KCl at pH 7.4 and pH 8.0 until the desired initial surface pressure (π) was reached. After the signal stabilized (∼5 min), 10 μg of protein was injected into the subphase through a hole in the wall of the trough. The change in surface pressure (Δπ) was monitored for 45 min. The Δπ value reached a maximum after 30 min in all experiments. The resulting Δπ was plotted versus π, and critical surface pressure (πχ) was determined as the x-intercept.
Surface Plasmon Resonance (SPR)
The SPR experiments were performed at 25 °C as described previously.19 Kinetic SPR measurements were carried out at a flow rate of 30 μl/min. After 90 μl of the protein in a particular pH buffer was injected, the association and dissociation were monitored for 90 s and over 500 s, respectively. The sensorgrams were obtained using five or more different concentrations of each protein within a 10-fold range of Kd and corrected for refractive index change by subtracting the control surface response. The association and dissociation phases of sensorgrams were globally fit to a 1:1 Langmuir binding model: protein + (protein binding site on vesicle) ↔ (complex) using BIAevalutation software (Biacore) as described previously.19 The dissociation constant (Kd) was calculated from the equation Kd = kd/ka. Each data set was repeated three times to obtain a standard deviation value. Mass transport37 was not a limiting factor in these experiments, since the change in a flow rate from 3 to 90 μl/min did not affect kinetics of association and dissociation. To verify the binding model, residual plots and c2 values were examined. Equilibrium binding measurements were performed at a 5 μl/min flow rate. The maximum response (Req) for each protein concentration was determined and plotted vs protein concentrations (C). The Kd value was determined by a nonlinear least squares analysis of the binding isotherm using the equation Req = Rmax/(1 + Kd/C).20
The in vivo localization of enhanced green fluorescent protein (EGFP)-fusion Epsin ENTH and AP180 ANTH domains
COS-1 cells were grown in Dulbecco Modified Essential Medium (DMEM) (Mediatech) supplied with 10% Fetal Bovine Serum (Invitrogen). The cells were plated on 25 mm glass coverslips and transfected with pEGFP-ANTH and pEGFP-ENTH using Effectene reagent (Qiagen). Cell imaging was performed 48-60 hours after transfection. Before imaging, cells were rinsed twice with Phosphate Buffered Saline (PBS: 137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4, pH 7.4) followed by addition of buffered solutions containing 10 μg/ml nigericin (Sigma) and 10 μg/ml monensin (Sigma). The buffered solutions contained 120 mM KCl, 20 mM NaCl, 0.5 mM CaCl2, 0.5 mM MgSO4, and phosphate or biphthalate buffers of pH 5.5-8.5. After equilibration for 15 min at 37°C, cells were fixed with 4% paraformaldehyde (Electron Microscopy Sciences) for 30 min at room temperature, rinsed once with the corresponding buffered solution and twice with PBS followed by permeabilization with 0.1% saponin (Acros Organics) in PBS for 10 min. The cells were then visualized on Olympus IX81 inverted microscope equipped with 60× oil immersion objective lens and a FITC filter set (Chroma), and operated by IPLab (v. 4.0) software (BD Biosciences).
Acknowledgements
We thank C. Burd, P. DeCamilli and A. Sorkin for discussions, P. DeCamilli and H. McMahon for providing cDNAs of the ENTH and ANTH domains and S. Lee for subcloning and mutagenesis. This research was supported by grants from the National Institutes of Health, GM070358 and GM073913 (to V.V.V) and GM071424 and CA113472 (to T.G.K.) and an Indiana University Biomedical Research Grant (to R.V.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brodsky FM, Chen CY, Knuehl C, Towler MC, Wakeham DE. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu Rev Cell Dev Biol. 2001;17:517–68. doi: 10.1146/annurev.cellbio.17.1.517. [DOI] [PubMed] [Google Scholar]
- 2.Sorkin A, Von Zastrow M. Signal transduction and endocytosis: close encounters of many kinds. Nat Rev Mol Cell Biol. 2002;3:600–14. doi: 10.1038/nrm883. [DOI] [PubMed] [Google Scholar]
- 3.Marsh M, McMahon HT. The structural era of endocytosis. Science. 1999;285:215–20. doi: 10.1126/science.285.5425.215. [DOI] [PubMed] [Google Scholar]
- 4.Cremona O, De Camilli P. Phosphoinositides in membrane traffic at the synapse. J Cell Sci. 2001;114:1041–52. doi: 10.1242/jcs.114.6.1041. [DOI] [PubMed] [Google Scholar]
- 5.Slepnev VI, De Camilli P. Accessory factors in clathrin-dependent synaptic vesicle endocytosis. Nat Rev Neurosci. 2000;1:161–72. doi: 10.1038/35044540. [DOI] [PubMed] [Google Scholar]
- 6.Ford MG, Pearse BM, Higgins MK, Vallis Y, Owen DJ, Gibson A, Hopkins CR, Evans PR, McMahon HT. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science. 2001;291:1051–5. doi: 10.1126/science.291.5506.1051. [DOI] [PubMed] [Google Scholar]
- 7.Itoh T, Koshiba S, Kigawa T, Kikuchi A, Yokoyama S, Takenawa T. Role of the ENTH domain in phosphatidylinositol-4,5-bisphosphate binding and endocytosis.[see comment] Science. 2001;291:1047–51. doi: 10.1126/science.291.5506.1047. [DOI] [PubMed] [Google Scholar]
- 8.Ford MG, Mills IG, Peter BJ, Vallis Y, Praefcke GJ, Evans PR, McMahon HT. Curvature of clathrin-coated pits driven by epsin.[comment] Nature. 2002;419:361–6. doi: 10.1038/nature01020. [DOI] [PubMed] [Google Scholar]
- 9.Stahelin RV, Long F, Peter BJ, Murray D, De Camilli P, McMahon HT, Cho W. Contrasting membrane interaction mechanisms of AP180 N-terminal homology (ANTH) and Epsin N-terminal homology (ENTH) domains. Journal of Biological Chemistry. 2003;278:28993–9. doi: 10.1074/jbc.M302865200. [DOI] [PubMed] [Google Scholar]
- 10.Itoh T, De Camilli P. BAR, F-BAR (EFC) and ENTH/ANTH domains in the regulation of membrane-cytosol interfaces and membrane curvature. Biochim Biophys Acta. 2006;1761:897–912. doi: 10.1016/j.bbalip.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 11.Legendre-Guillemin V, Wasiak S, Hussain NK, Angers A, McPherson PS. ENTH/ANTH proteins and clathrin-mediated membrane budding. J Cell Sci. 2004;117:9–18. doi: 10.1242/jcs.00928. [DOI] [PubMed] [Google Scholar]
- 12.Rosenthal JA, Chen H, Slepnev VI, Pellegrini L, Salcini AE, Di Fiore PP, De Camilli P. The epsins define a family of proteins that interact with components of the clathrin coat and contain a new protein module. J Biol Chem. 1999;274:33959–65. doi: 10.1074/jbc.274.48.33959. [DOI] [PubMed] [Google Scholar]
- 13.Kay BK, Yamabhai M, Wendland B, Emr SD. Identification of a novel domain shared by putative components of the endocytic and cytoskeletal machinery. Protein Sci. 1999;8:435–8. doi: 10.1110/ps.8.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen H, Fre S, Slepnev VI, Capua MR, Takei K, Butler MH, Di Fiore PP, De Camilli P. Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature. 1998;394:793–7. doi: 10.1038/29555. [DOI] [PubMed] [Google Scholar]
- 15.Drake MT, Downs MA, Traub LM. Epsin binds to clathrin by associating directly with the clathrin-terminal domain. Evidence for cooperative binding through two discrete sites. J Biol Chem. 2000;275:6479–89. doi: 10.1074/jbc.275.9.6479. [DOI] [PubMed] [Google Scholar]
- 16.Yamabhai M, Hoffman NG, Hardison NL, McPherson PS, Castagnoli L, Cesareni G, Kay BK. Intersectin, a novel adaptor protein with two Eps15 homology and five Src homology 3 domains. J Biol Chem. 1998;273:31401–7. doi: 10.1074/jbc.273.47.31401. [DOI] [PubMed] [Google Scholar]
- 17.Hyman J, Chen H, Di Fiore PP, De Camilli P, Brunger AT. Epsin 1 undergoes nucleocytosolic shuttling and its eps15 interactor NH(2)-terminal homology (ENTH) domain, structurally similar to Armadillo and HEAT repeats, interacts with the transcription factor promyelocytic leukemia Zn(2)+ finger protein (PLZF) J Cell Biol. 2000;149:537–46. doi: 10.1083/jcb.149.3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mao Y, Chen J, Maynard JA, Zhang B, Quiocho FA. A novel all helix fold of the AP180 amino-terminal domain for phosphoinositide binding and clathrin assembly in synaptic vesicle endocytosis. Cell. 2001;104:433–40. doi: 10.1016/s0092-8674(01)00230-6. [DOI] [PubMed] [Google Scholar]
- 19.Stahelin RV, Cho W. Differential role of ionic, aliphatic, and aromatic residues in membrane-protein interactions: a surface plasmon resonance study on phospholipases A2. Biochemistry. 2001;40:4672–8. doi: 10.1021/bi0020325. [DOI] [PubMed] [Google Scholar]
- 20.Stahelin RV, Long F, Diraviyam K, Bruzik KS, Murray D, Cho W. Phosphatidylinositol 3-phosphate induces the membrane penetration of the FYVE domains of Vps27p and Hrs. J. Biol. Chem. 2002;277:26379–88. doi: 10.1074/jbc.M201106200. [DOI] [PubMed] [Google Scholar]
- 21.Llopis J, McCaffery JM, Miyawaki A, Farquhar MG, Tsien RY. Measurement of cytosolic, mitochondrial, and Golgi pH in single living cells with green fluorescent proteins. Proc Natl Acad Sci U S A. 1998;95:6803–8. doi: 10.1073/pnas.95.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brett CL, Tukaye DN, Mukherjee S, Rao R. The Yeast Endosomal Na+(K+)/H+ Exchanger Nhx1 Regulates Cellular pH to Control Vesicle Trafficking. Mol Biol Cell. 2005;16:1396–405. doi: 10.1091/mbc.E04-11-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osborne SL, Thomas CL, Gschmeissner S, Schiavo G. Nuclear PtdIns(4,5)P2 assembles in a mitotically regulated particle involved in pre-mRNA splicing. J Cell Sci. 2001;114:2501–11. doi: 10.1242/jcs.114.13.2501. [DOI] [PubMed] [Google Scholar]
- 24.Lee SA, Eyeson R, Cheever ML, Geng J, Verkhusha VV, Burd C, Overduin M, Kutateladze TG. Targeting of the FYVE domain to endosomal membranes is regulated by a histidine switch. Proc Natl Acad Sci U S A. 2005;102:13052–7. doi: 10.1073/pnas.0503900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Paridon PA, de Kruijff B, Ouwerkerk R, Wirtz KW. Polyphosphoinositides undergo charge neutralization in the physiological pH range: a 31P-NMR study. Biochim Biophys Acta. 1986;877:216–9. doi: 10.1016/0005-2760(86)90137-2. [DOI] [PubMed] [Google Scholar]
- 26.Hildebrand J, Marique D, Vanhouche J. Lipid composition of plasma membranes from human leukemic lymphocytes. J Lipid Res. 1975;16:195–9. [PubMed] [Google Scholar]
- 27.Stahelin RV, Burian A, Bruzik KS, Murray D, Cho W. Membrane binding mechanisms of the PX domains of NADPH oxidase p40phox and p47phox. Journal of Biological Chemistry. 2003;278:14469–79. doi: 10.1074/jbc.M212579200. [DOI] [PubMed] [Google Scholar]
- 28.Kutateladze TG, Capelluto DGS, Ferguson CG, Cheever ML, Kutateladze AG, Prestwich GD, Overduin M. Multivalent mechanism of membrane insertion by the FYVE domain. J. Biol. Chem. 2004;279:3050–7. doi: 10.1074/jbc.M309007200. [DOI] [PubMed] [Google Scholar]
- 29.Marsh D. Lateral pressure in membranes. Biochim Biophys Acta. 1996;1286:183–223. doi: 10.1016/s0304-4157(96)00009-3. [DOI] [PubMed] [Google Scholar]
- 30.Gottlieb RA, Giesing HA, Zhu JY, Engler RL, Babior BM. Cell acidification in apoptosis: granulocyte colony-stimulating factor delays programmed cell death in neutrophils by up-regulating the vacuolar H(+)-ATPase. Proc Natl Acad Sci U S A. 1995;92:5965–8. doi: 10.1073/pnas.92.13.5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moolenaar WH, Tsien RY, van der Saag PT, de Laat SW. Na+/H+ exchange and cytoplasmic pH in the action of growth factors in human fibroblasts. Nature. 1983;304:645–8. doi: 10.1038/304645a0. [DOI] [PubMed] [Google Scholar]
- 32.Schuldiner S, Rozengurt E. Na+/H+ antiport in Swiss 3T3 cells: mitogenic stimulation leads to cytoplasmic alkalinization. Proc Natl Acad Sci U S A. 1982;79:7778–82. doi: 10.1073/pnas.79.24.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thangaraju M, Sharma K, Leber B, Andrews DW, Shen SH, Srikant CB. Regulation of acidification and apoptosis by SHP-1 and Bcl-2. J Biol Chem. 1999;274:29549–57. doi: 10.1074/jbc.274.41.29549. [DOI] [PubMed] [Google Scholar]
- 34.LaManna JC. Hypoxia/ischemia and the pH paradox. Adv. Exp. Med. Biol. 1996;388:283–92. doi: 10.1007/978-1-4613-0333-6_36. [DOI] [PubMed] [Google Scholar]
- 35.Punnia-Moorthy A. Evaluation of pH changes in inflammation of the subcutaneous air pouch lining in the rat, induced by carrageenan, dextran and Staphylococcus aureus. J. Oral Pathology. 1987;16:36–44. doi: 10.1111/j.1600-0714.1987.tb00674.x. [DOI] [PubMed] [Google Scholar]
- 36.Gerweck LE, Seetharaman K. Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer. Cancer Res. 1996;56:1194–8. [PubMed] [Google Scholar]
- 37.Myszka DG, Morton TA, Doyle ML, Chaiken IM. Kinetic analysis of a protein antigen-antibody interaction limited by mass transport on an optical biosensor. Biophys Chem. 1997;64:127–37. doi: 10.1016/s0301-4622(96)02230-2. [DOI] [PubMed] [Google Scholar]