Abstract
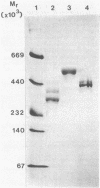
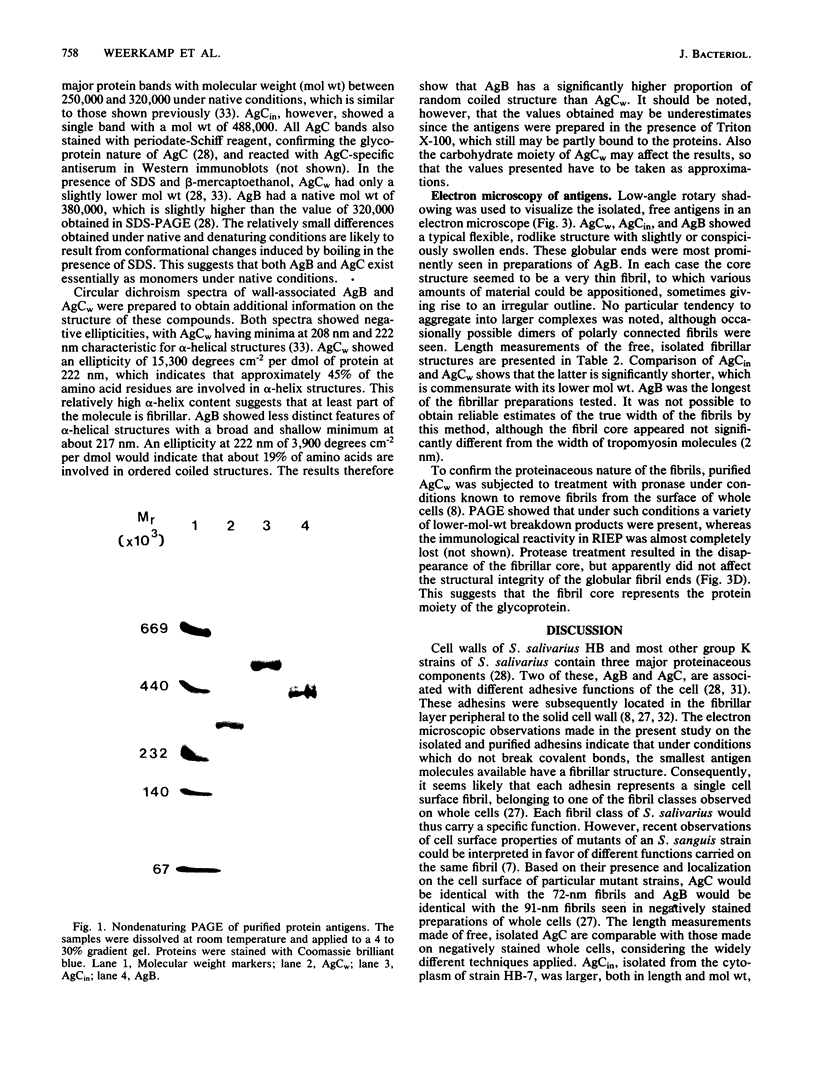
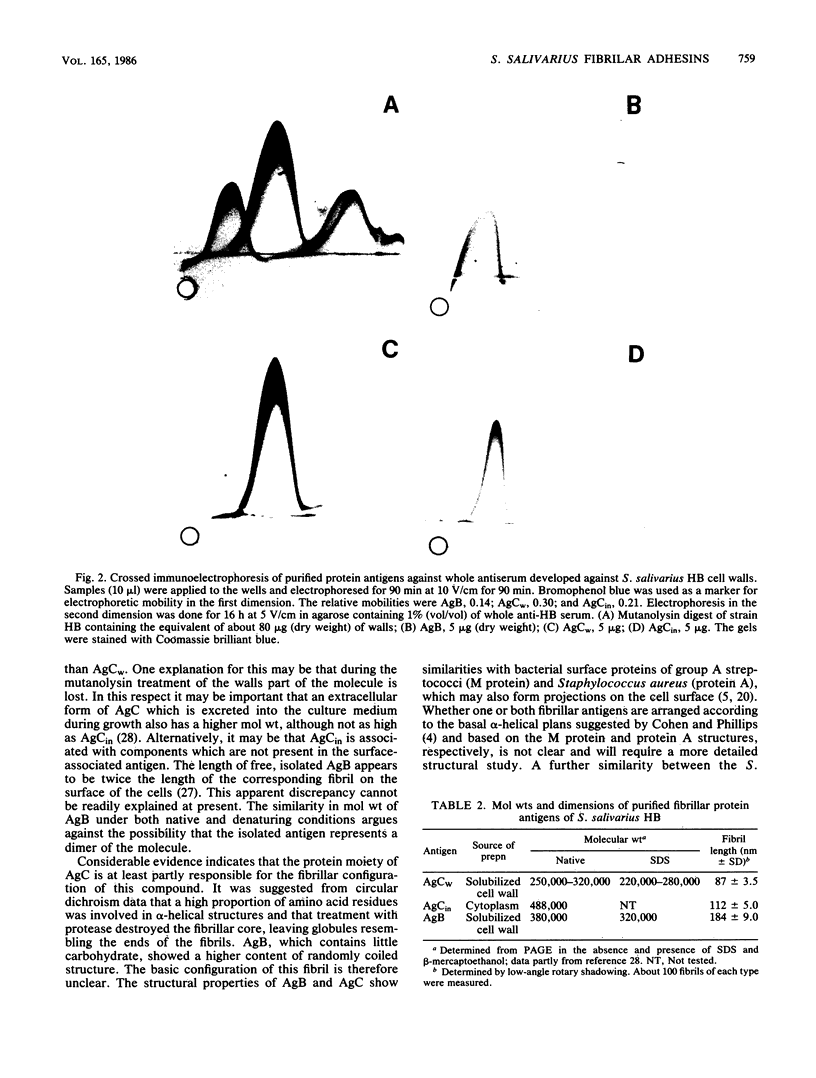
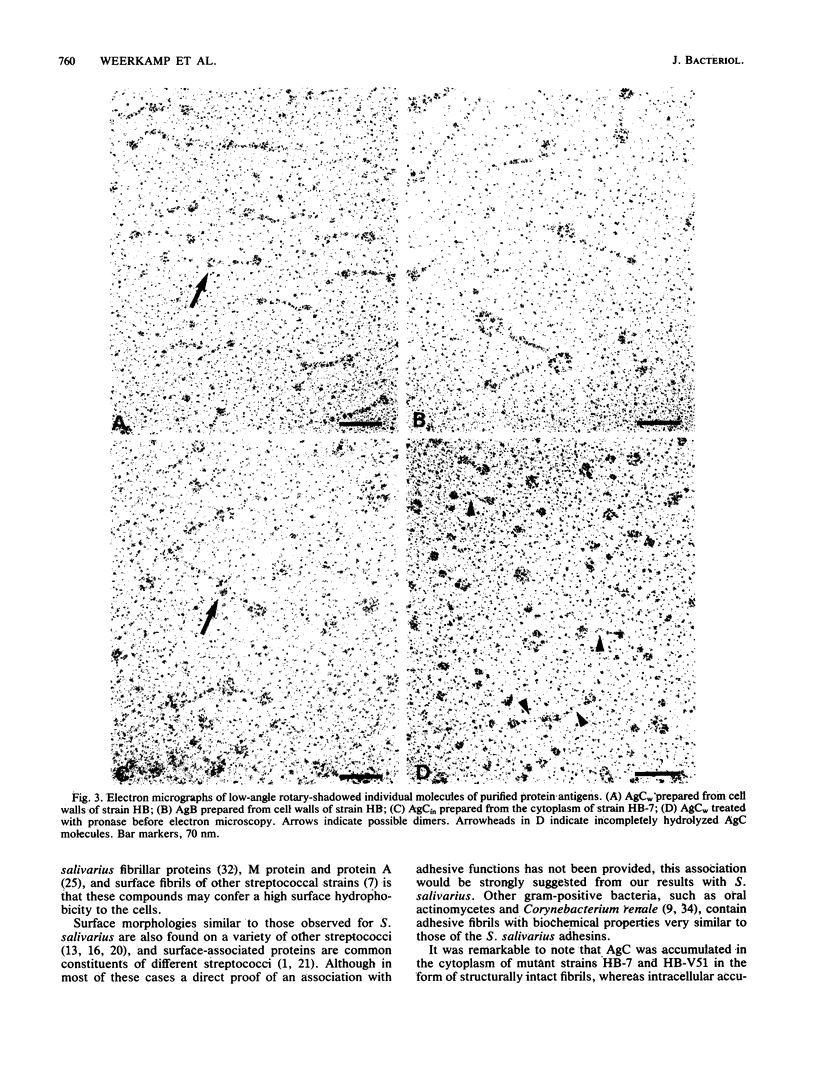
Most Streptococcus salivarius (K+) cells contain two protein antigens with different adhesive functions. The subcellular distribution and some structural properties of purified proteins were studied. Antigen B (AgB), a protein involved in interbacterial coaggregation with gram-negative bacteria, was present in the cell wall fraction only of the wild-type strain and was absent from the cells of a nonadhesive mutant. Antigen C (AgC), a glycoprotein involved in host-associated adhesive functions, was predominantly associated with the cell wall of the wild-type strain (AgCw), but accumulated in high amounts in the cytoplasmic fraction (AgCin) of mutants lacking the wall-associated form. AgB, AgCw, and AgCin had molecular weights of 380,000, 250,000 to 320,000, and 488,000, respectively, upon gel electrophoresis under nondenaturing conditions. In the presence of sodium dodecyl sulfate and beta-mercaptoethanol the molecular weights were only slightly lower, suggesting that the free, isolated molecules exist as monomers under native conditions. AgCin readily stained with periodate-Schiff reagent, indicating a significant content of carbohydrate, similar to AgCw. Circular dichroism spectra showed that about 45% of the amino acids of AgCw were involved in alpha-helical coiled structures. AgB had a significantly lower proportion of ordered coiled structure. Electron microscopic observations of low-angle-shadowed preparations of purified antigens showed that they were flexible, thin rods with thickened or globular ends. Measurements corrected for shadow thickness showed lengths of 184 nm (AgB), 112 nm (AgCin), and 87 nm (AgCw). Treatment of AgCw with protease destroyed the fibrillar core, but seemed not to affect the globular ends. Comparison of the results with the localization of the antigens in wild-type and specific mutant strains suggested that each antigen molecule may represent a single, characteristic surface fibril with a specific adhesive capacity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelbaum B., Rosan B. Cell surface proteins of oral streptococci. Infect Immun. 1984 Oct;46(1):245–250. doi: 10.1128/iai.46.1.245-250.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Cisar J. O., Sandberg A. L., Mergenhagen S. E. The function and distribution of different fimbriae on strains of Actinomyces viscosus and Actinomyces naeslundii. J Dent Res. 1984 Mar;63(3):393–396. doi: 10.1177/00220345840630030701. [DOI] [PubMed] [Google Scholar]
- Cohen C., Phillips G. N., Jr Spikes and fimbriae: alpha-helical proteins form surface projections on microorganisms. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5303–5304. doi: 10.1073/pnas.78.9.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisenhofer J., Jones T. A., Huber R., Sjödahl J., Sjöquist J. Crystallization, crystal structure analysis and atomic model of the complex formed by a human Fc fragment and fragment B of protein A from Staphylococcus aureus. Hoppe Seylers Z Physiol Chem. 1978 Aug;359(8):975–985. doi: 10.1515/bchm2.1978.359.2.975. [DOI] [PubMed] [Google Scholar]
- Douglas C. W., Russell R. R. The adsorption of human salivary components to strains of the bacterium Streptococcus mutans. Arch Oral Biol. 1984;29(10):751–757. doi: 10.1016/0003-9969(84)90002-5. [DOI] [PubMed] [Google Scholar]
- Fives-Taylor P. M., Thompson D. W. Surface properties of Streptococcus sanguis FW213 mutants nonadherent to saliva-coated hydroxyapatite. Infect Immun. 1985 Mar;47(3):752–759. doi: 10.1128/iai.47.3.752-759.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley P. S., Carter P. L., Fielding J. Streptococcus salivarius strains carry either fibrils or fimbriae on the cell surface. J Bacteriol. 1984 Jan;157(1):64–72. doi: 10.1128/jb.157.1.64-72.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda E., Yanagawa R. Agglutination of trypsinized sheep erythrocytes by the pili of Corynebacterium renale. Infect Immun. 1974 Dec;10(6):1426–1432. doi: 10.1128/iai.10.6.1426-1432.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Isaacson R. E. Proteinaceous bacterial adhesins and their receptors. Crit Rev Microbiol. 1983;10(3):229–260. doi: 10.3109/10408418209113564. [DOI] [PubMed] [Google Scholar]
- Kolenbrander P. E., Williams B. L. Lactose-reversible coaggregation between oral actinomycetes and Streptococcus sanguis. Infect Immun. 1981 Jul;33(1):95–102. doi: 10.1128/iai.33.1.95-102.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Gibbons R. J. Proportional distribution and relative adherence of Streptococcus miteor (mitis) on various surfaces in the human oral cavity. Infect Immun. 1972 Nov;6(5):852–859. doi: 10.1128/iai.6.5.852-859.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride B. C., Song M., Krasse B., Olsson J. Biochemical and immunological differences between hydrophobic and hydrophilic strains of Streptococcus mutans. Infect Immun. 1984 Apr;44(1):68–75. doi: 10.1128/iai.44.1.68-75.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Nakao M., Shibata S., Shizukuishi S., Nakamura R., Tsunemitsu A. Purification and characterization of galactosephilic component present on the cell surfaces of Streptococcus sanguis ATCC 10557. J Periodontol. 1983 Mar;54(3):163–172. doi: 10.1902/jop.1983.54.3.163. [DOI] [PubMed] [Google Scholar]
- Nalbandian J., Freedman M. L., Tanzer J. M., Lovelace S. M. Ultrastructure of Mutants of Streptococcus mutans with Reference to Agglutination, Adhesion, and Extracellular Polysaccharide. Infect Immun. 1974 Nov;10(5):1170–1179. doi: 10.1128/iai.10.5.1170-1179.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottow J. C. Ecology, physiology, and genetics of fimbriae and pili. Annu Rev Microbiol. 1975;29:79–108. doi: 10.1146/annurev.mi.29.100175.000455. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Phillips G. N., Jr, Flicker P. F., Cohen C., Manjula B. N., Fischetti V. A. Streptococcal M protein: alpha-helical coiled-coil structure and arrangement on the cell surface. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4689–4693. doi: 10.1073/pnas.78.8.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shotton D. M., Burke B. E., Branton D. The molecular structure of human erythrocyte spectrin. Biophysical and electron microscopic studies. J Mol Biol. 1979 Jun 25;131(2):303–329. doi: 10.1016/0022-2836(79)90078-0. [DOI] [PubMed] [Google Scholar]
- Simpson W. A., Beachey E. H. Adherence of group A streptococci to fibronectin on oral epithelial cells. Infect Immun. 1983 Jan;39(1):275–279. doi: 10.1128/iai.39.1.275-279.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinglu G., Ghosh A., Ghosh B. K. Subcellular localization of alkaline phosphatase in Bacillus licheniformis 749/C by immunoelectron microscopy with colloidal gold. J Bacteriol. 1984 Aug;159(2):668–677. doi: 10.1128/jb.159.2.668-677.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerkamp A. H., Handley P. S., Baars A., Slot J. W. Negative staining and immunoelectron microscopy of adhesion-deficient mutants of Streptococcus salivarius reveal that the adhesive protein antigens are separate classes of cell surface fibril. J Bacteriol. 1986 Mar;165(3):746–755. doi: 10.1128/jb.165.3.746-755.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerkamp A. H., Jacobs T. Cell wall-associated protein antigens of Streptococcus salivarius: purification, properties, and function in adherence. Infect Immun. 1982 Oct;38(1):233–242. doi: 10.1128/iai.38.1.233-242.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerkamp A. H., McBride B. C. Identification of a Streptococcus salivarius cell wall component mediating coaggregation with Veillonella alcalescens V1. Infect Immun. 1981 May;32(2):723–730. doi: 10.1128/iai.32.2.723-730.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler T. T., Clark W. B. Fibril-mediated adherence of Actinomyces viscosus to saliva-treated hydroxyapatite. Infect Immun. 1980 May;28(2):577–584. doi: 10.1128/iai.28.2.577-584.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]