Abstract
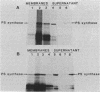
To better establish the intracellular location of the phosphatidylserine synthase of Escherichia coli and hence better understand how it is regulated in the cell, we compared the size, function, and binding properties of the enzyme made in vitro with the enzyme found in cell lysates and with the purified enzyme. The enzyme made either in vivo or in an active form in vitro was found primarily associated with the ribosomal fraction of the cell and had the same apparent molecular mass as the purified enzyme. These results were unaffected by the presence of protease inhibitors. Addition of unsupplemented E. coli membranes or membranes supplemented with phosphatidylethanolamine did not affect the subcellular distribution of the enzyme in these experiments. However, addition of membranes supplemented with either the lipid substrate, CDP-diacylglycerol, or the lipid product, phosphatidylserine, resulted in membrane association by the enzyme rather than ribosomal association. Addition of membranes supplemented with acidic lipids also brought about membrane association, but this association was primarily ionic since it was disrupted by high salt concentrations. These results strongly suggest that the ribosomal location of this enzyme is not the result of some modification event occurring after cell lysis and that the normal functioning of the enzyme involves membrane association which is primarily induced by the presence of a membrane-associated substrate.
Full text
PDF

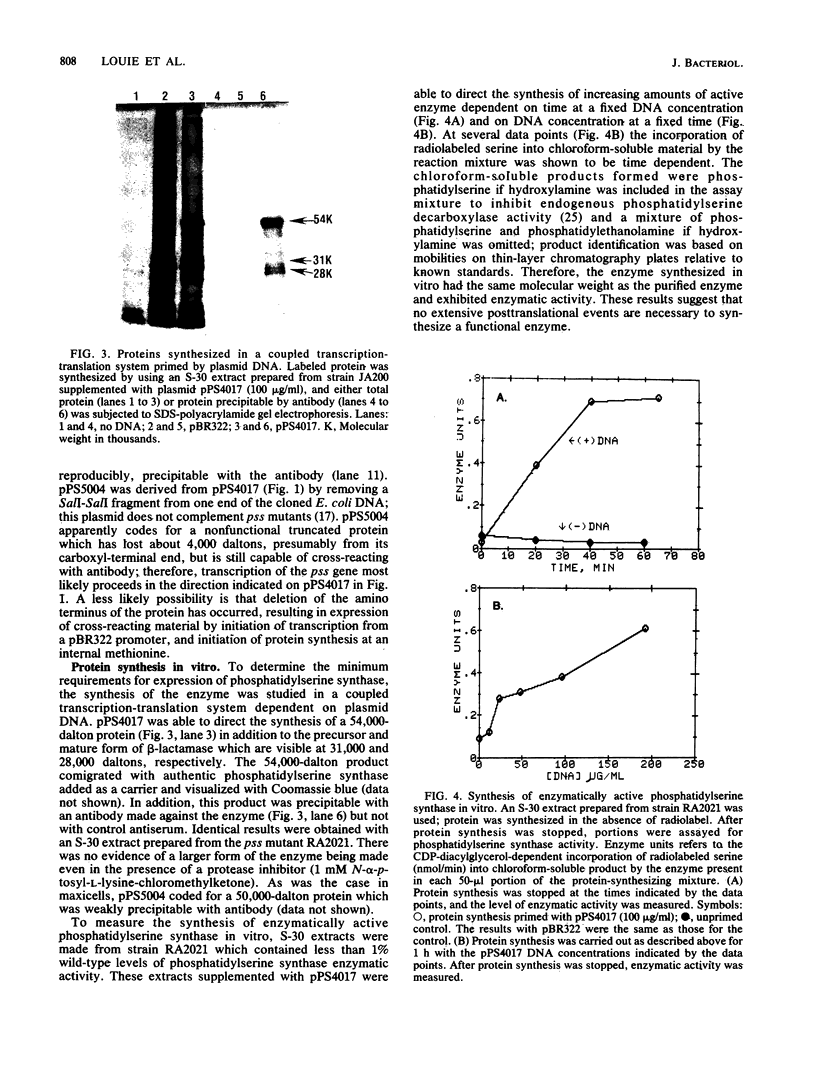
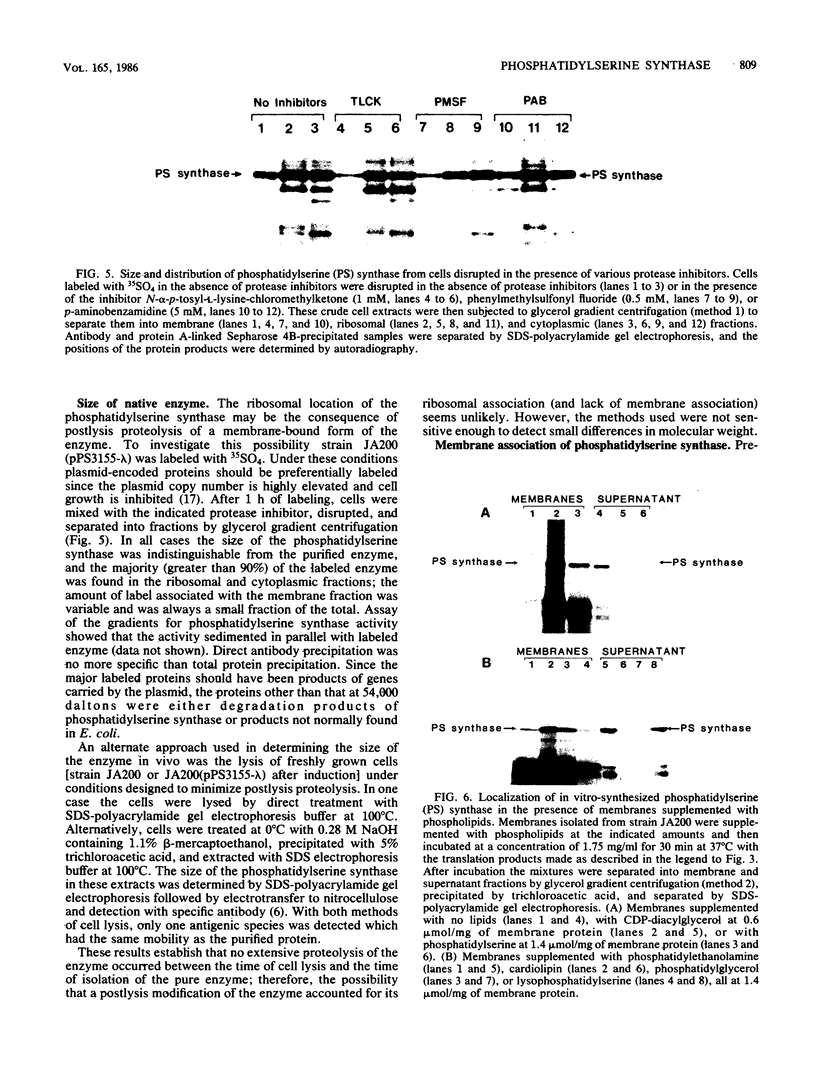
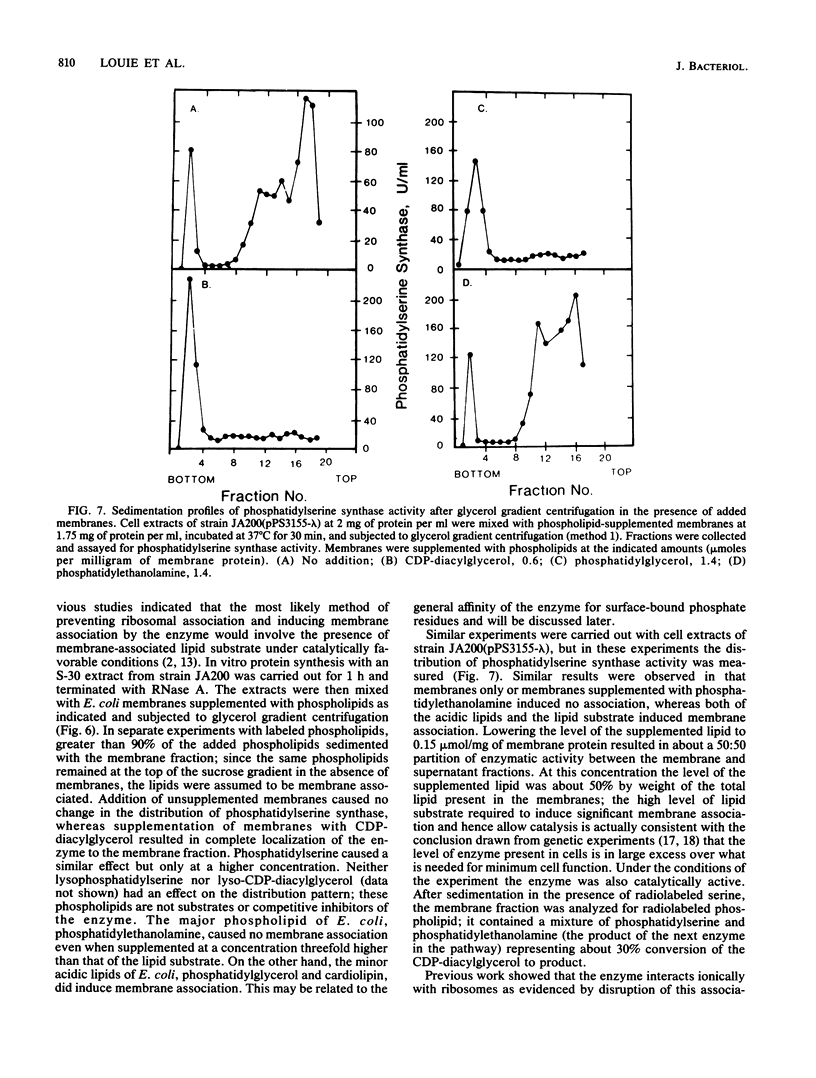
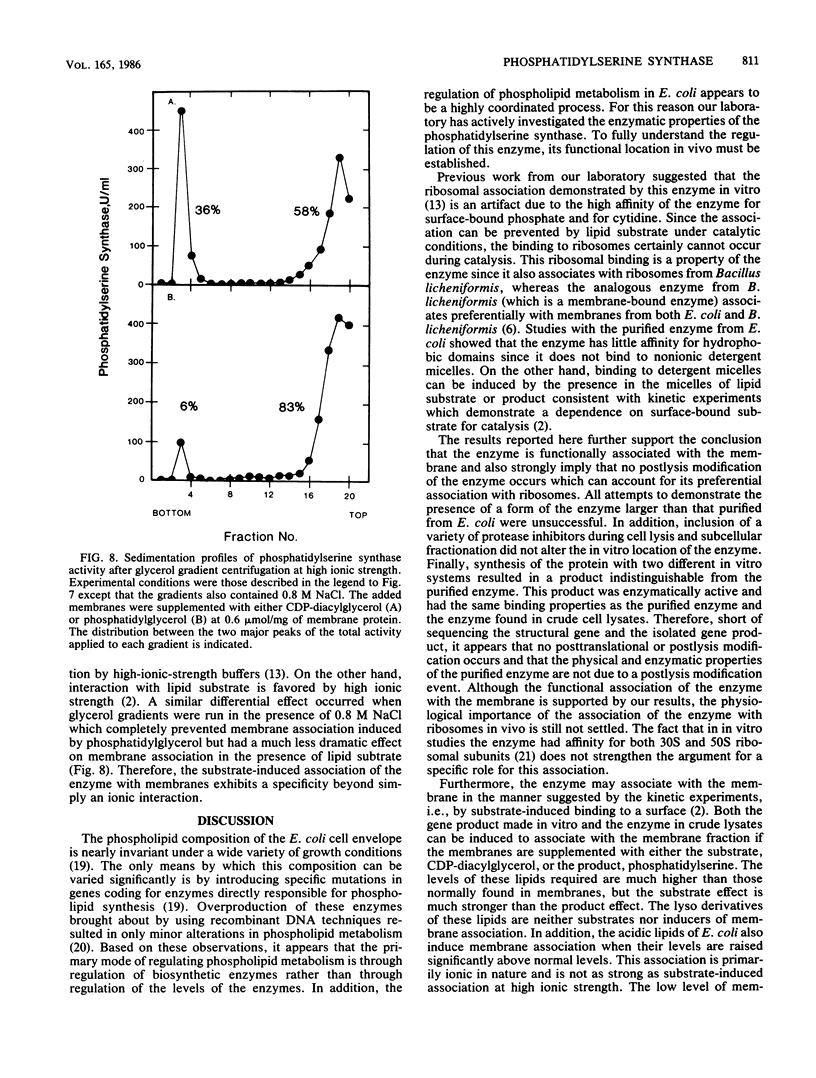

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes W. M. Plasmid detection and sizing in single colony lysates. Science. 1977 Jan 28;195(4276):393–394. doi: 10.1126/science.318764. [DOI] [PubMed] [Google Scholar]
- Carman G. M., Dowhan W. Phosphatidylserine synthase from Escherichia coli. The role of Triton X-100 in catalysis. J Biol Chem. 1979 Sep 10;254(17):8391–8397. [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Dutt A., Dowhan W. Characterization of a membrane-associated cytidine diphosphate-diacylglycerol-dependent phosphatidylserine synthase in bacilli. J Bacteriol. 1981 Aug;147(2):535–542. doi: 10.1128/jb.147.2.535-542.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutt A., Dowhan W. Intracellular distribution of enzymes of phospholipid metabolism in several gram-negative bacteria. J Bacteriol. 1977 Oct;132(1):159–165. doi: 10.1128/jb.132.1.159-165.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutt A., Dowhan W. Purification and characterization of a membrane-associated phosphatidylserine synthase from Bacillus licheniformis. Biochemistry. 1985 Feb 26;24(5):1073–1079. doi: 10.1021/bi00326a001. [DOI] [PubMed] [Google Scholar]
- Firestone G. L., Heath E. C. The cyclic AMP-mediated induction of alkaline phosphatase in mouse L-cells. J Biol Chem. 1981 Feb 10;256(3):1396–1403. [PubMed] [Google Scholar]
- Hawrot E., Kennedy E. P. Phospholipid composition and membrane function in phosphatidylserine decarboxylase mutants of Escherichia coli. J Biol Chem. 1978 Nov 25;253(22):8213–8220. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larson T. J., Dowhan W. Ribosomal-associated phosphatidylserine synthetase from Escherichia coli: purification by substrate-specific elution from phosphocellulose using cytidine 5'-diphospho-1,2-diacyl-sn-glycerol. Biochemistry. 1976 Nov 30;15(24):5212–5218. doi: 10.1021/bi00669a003. [DOI] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie K., Dowhan W. Investigations on the association of phosphatidylserine synthase with the ribosomal component from Escherichia coli. J Biol Chem. 1980 Feb 10;255(3):1124–1127. [PubMed] [Google Scholar]
- McMurry L., Petrucci R. E., Jr, Levy S. B. Active efflux of tetracycline encoded by four genetically different tetracycline resistance determinants in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3974–3977. doi: 10.1073/pnas.77.7.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki C., Kuroda M., Ohta A., Shibuya I. Genetic manipulation of membrane phospholipid composition in Escherichia coli: pgsA mutants defective in phosphatidylglycerol synthesis. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7530–7534. doi: 10.1073/pnas.82.22.7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta A., Waggoner K., Louie K., Dowhan W. Cloning of genes involved in membrane lipid synthesis. Effects of amplification of phosphatidylserine synthase in Escherichia coli. J Biol Chem. 1981 Mar 10;256(5):2219–2225. [PubMed] [Google Scholar]
- Raetz C. R., Kantor G. D., Nishijima M., Newman K. F. Cardiolipin accumulation in the inner and outer membranes of Escherichia coli mutants defective in phosphatidylserine synthetase. J Bacteriol. 1979 Aug;139(2):544–551. doi: 10.1128/jb.139.2.544-551.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R., Kennedy E. P. The association of phosphatidylserine synthetase with ribosomes in extracts of Escherichia coli. J Biol Chem. 1972 Apr 10;247(7):2008–2014. [PubMed] [Google Scholar]
- Raetz C. R., Larson T. J., Dowhan W. Gene cloning for the isolation of enzymes of membrane lipid synthesis: phosphatidylserine synthase overproduction in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1412–1416. doi: 10.1073/pnas.74.4.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R. Phosphatidylserine synthetase mutants of Escherichia coli. Genetic mapping and membrane phospholipid composition. J Biol Chem. 1976 Jun 10;251(11):3242–3249. [PubMed] [Google Scholar]
- Rupp W. D., Wilde C. E., 3rd, Reno D. L., Howard-Flanders P. Exchanges between DNA strands in ultraviolet-irradiated Escherichia coli. J Mol Biol. 1971 Oct 14;61(1):25–44. doi: 10.1016/0022-2836(71)90204-x. [DOI] [PubMed] [Google Scholar]
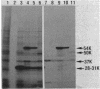
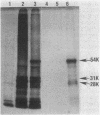
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satre M., Kennedy E. P. Identification of bound pyruvate essential for the activity of phosphatidylserine decarboxylase of Escherichia coli. J Biol Chem. 1978 Jan 25;253(2):479–483. [PubMed] [Google Scholar]
- Zubay G. In vitro synthesis of protein in microbial systems. Annu Rev Genet. 1973;7:267–287. doi: 10.1146/annurev.ge.07.120173.001411. [DOI] [PubMed] [Google Scholar]