Abstract
Increased spontaneous mutation is associated with increased cancer risk. Here, by using a model system, we show that spontaneous mutation can be increased several hundred-fold by a simple imbalance between the first two enzymes involved in DNA base excision repair. The Saccharomyces cerevisiae MAG1 3-methyladenine (3MeA) DNA glycosylase, when expressed at high levels relative to the apurinic/apyrimidinic endonuclease, increases spontaneous mutation by up to ≈600-fold in S. cerevisiae and ≈200-fold in Escherichia coli. Genetic evidence suggests that, in yeast, the increased spontaneous mutation requires the generation of abasic sites and the processing of these sites by the REV1/REV3/REV7 lesion bypass pathway. Comparison of the mutator activity produced by Mag1, which has a broad substrate range, with that produced by the E. coli Tag 3MeA DNA glycosylase, which has a narrow substrate range, indicates that the removal of endogenously produced 3MeA is unlikely to be responsible for the mutator effect of Mag1. Finally, the human AAG 3-MeA DNA glycosylase also can produce a small (≈2-fold) but statistically significant increase in spontaneous mutation, a result which could have important implications for carcinogenesis.
Keywords: 3-MeA DNA glycosylase/AP endonuclease/REV genes/cancer risk
Mutation is a characteristic of all living organisms and can arise from a variety of sources. DNA is chemically unstable and its replication imperfect; such instability and infidelity can generate mutations. In addition, a variety of endogenous metabolites and exogenous physical and chemical agents can generate potentially mutagenic DNA adducts. Despite these threats organisms maintain a relatively low mutation rate, due in part to a diverse group of DNA repair pathways (1). Defects in several kinds of DNA repair are known to elevate spontaneous mutation (2, 3), and certain mammalian DNA repair defects that influence spontaneous mutation result in remarkably increased cancer risk (4).
Subtly altered DNA bases, such as deaminated, oxidized, and alkylated bases, are repaired by the highly conserved base excision repair pathway (5, 6). The first step of this multistep pathway involves the release of a modified DNA base by a specific DNA glycosylase. The resulting abasic site represents another form of DNA damage that is potentially cytotoxic and mutagenic (7, 8). Abasic sites are processed, in most cases, by apurinic/apyrimidinic (AP) endonucleases that cleave DNA 5′ to the abasic site, generating a single strand break that cannot be ligated. The abasic sugar residue is removed by deoxyribophosphodiesterase, the gap filled by DNA polymerase, and the remaining nick sealed by DNA ligase (5).
In S. cerevisiae, the 3MeA DNA glycosylase encoded by MAG1 (9) is the major activity for removing alkylated bases. Mag1 releases a variety of bases from DNA in vitro, including 3MeA, 7-methylguanine, 7-methyladenine, 3-methylguanine (9, 10), 7-hydroxyethylguanine, 7-chloroethylguanine (11), 1,N6-ethenoadenine (12), and hypoxanthine (deaminated adenine) (13). The abasic sites generated by Mag1 are processed normally by the major yeast APN1-encoded AP endonuclease (14, 15). The mutagenic potential of abasic sites is demonstrated clearly by the fact that apn1 mutants have elevated spontaneous mutation rates (16, 17). This mutator phenotype is influenced by Mag1 levels: decreased Mag1 expression suppresses, and increased expression enhances, the mutator phenotype (17, 18).
Here, we explore how Mag1 modulates spontaneous mutation. We show that Mag1 expression in yeast produces a 20- to 600-fold increase in spontaneous mutation rate, depending on the Mag1:Apn1 ratio. We also provide evidence that the mutator effect of Mag1 depends on the production of abasic sites and that these abasic sites are converted into mutations by the REV1/REV3/REV7 lesion bypass system (19, 20). In addition, we show that the Mag1 human homolog AAG modestly elevates spontaneous mutation in S. cerevisiae and that the 3MeA repair capacity of Mag1 and AAG is probably not responsible for their mutator activity. High ratios of Mag1 and AAG to Apn1 also are associated with sensitivity to cell killing by methylating agents. The potential for DNA glycosylases to generate a mutator phenotype may have important implications for cancer susceptibility and carcinogenesis.
MATERIALS AND METHODS
Plasmids.
For 3MeA DNA glycosylase expression in yeast and bacteria the E. coli tag-, the S. cerevisiae MAG1-, and the human AAG-coding sequences were cloned into the galactose inducible pYES2.0 yeast expression vector (pYES, Invitrogen) and subsequently into the arabinose inducible pBAD24 bacterial expression vector [(21), provided by Jon Beckwith, Harvard Medical School, Boston]. pYES-tag, pYES-MAG, and pYES-AAG were checked for their ability to complement the methyl methanesulfonate (MMS)-sensitive phenotype of a mag1-deletion strain (BGY148, Table 1) by using MMS gradient plates (22) and by DNA sequencing of each coding sequence. All three constructs complemented the MMS sensitivity of BGY148 (data not shown). The AAG sequence in pYES-AAG has a single base pair change resulting in an L83H amino acid substitution. However, pYES-AAG produces robust 3MeA DNA glycosylase activity and fully complements the MMS sensitivity of BGY148 (see later). Plasmid YEpAPN1 containing the yeast APN1 gene (23) was provided by Bruce Demple (Harvard School of Public Health, Boston).
Table 1.
Strains
| Strain | Genotype |
|---|---|
| S. cerevisiae | |
| DBY747 | MATa his3-Δ1 leu2-3,112 ura3-52 trp1-289a can1 galS CUPr |
| FY86 | MATa his3-Δ200 ura3-52 leu2-Δ1 GAL+ |
| BGY111 | MATa his3 leu2 ura3-52 trp1-289a can1 GAL+ |
| BGY113 | BGY111 apn1-Δ1∷HIS3 |
| BGY148 | BGY111 mag1-Δ2∷LEU2 |
| BGY149 | BGY111 apn1-Δ1∷HIS3 mag1-Δ2∷LEU2 |
| BGY167 | BGY149 rev1-Δ∷hisG |
| BGY168 | BGY149 rev3-Δ∷hisG |
| BGY169 | BGY149 rev7-Δ∷hisG |
| E. coli | |
| AB1157 | F−thr-1 leu-6 proA2 thi-1 argE lacY1 galK ara-14 xyl-5 mtl-1 tsx-33 strA sup-37 |
| BW528 | AB1157 Δxth nfol∷kan |
Strains.
Table 1 lists the strains used in this study. DBY747 (Eric Eisenstadt, Office of Naval Research, Washington, DC) and FY86 (Fred Winston, Harvard Medical School, Boston) were mated and then used to generate the Gal+ Trp− haploid segregant BGY111. Strain BGY113 was generated from BGY111 by one-step gene replacement with a 3.2-kb EcoRI/BamHI fragment of pSCP19A (16) containing an apn1-deletion/disruption cassette. BGY148 (Leu+ Mag−) was generated similarly by using a mag1-deletion/disruption cassette. BGY149 was derived from BGY113 by using the same procedure. The mag1-deletion derivatives were verified by Southern analysis (24) (data not shown). Strains BGY167, −168, and −169 were constructed by using deletion/disruption “gene blaster” cassettes (25). Plasmids pSF3 (rev1-Δ cassette), pYPG101 (rev3-Δ cassette), and pYPG102 (rev7-Δ cassette) (all constructed and generously provided by Christopher Lawrence, University of Rochester, Rochester, NY) were linearized; BGY149 was transformed with each cassette to generate strains BGY167, −168, and −169, respectively. Ura+ Rev− transformants were identified by a moderate UV sensitivity and a marked decrease in UV-induced mutation (26) at the trp1–289a locus (data not shown). The URA3 markers were recycled (25) by selection on 5-fluoroorotic acid (27). Plasmid pSCP19A was kindly provided by Richard Bennett and Bruce Demple (Harvard School of Public Health, Boston). Bacterial strain BW528 (28) was provided by Bruce Demple.
Measuring the 3MeA DNA Glycosylase Activity in Bacteria and Yeast Extracts.
3MeA DNA glycosylase activity was determined by incubation of crude cell extract proteins with calf thymus DNA alkylated with 3H-CH2-methylnitrosourea (3.6 Ci/mmol; 1 Ci = 37 GBq), followed by chromatographic separation of the glycosylase-released bases, as described (9). Bacteria and yeast were grown and induced as described below, and cell extracts were prepared by using standard techniques (29).
Determining Spontaneous Mutation Rates for Yeast.
The spontaneous mutation rate of yeast strains using the trp1–289a locus was determined by fluctuation analysis essentially as described (17, 30) by using the fraction of cultures showing no mutation (Po method, ref. 31) except where noted. In brief, cultures (5 ml) were grown to saturation (1–3 × 107 cells/ml) at 30°C in synthetic dextrose minimal medium (32) lacking uracil (to maintain selection of plasmids) (SD-URA) containing 2% glucose and the required amino acids for each auxotrophic strain at standard concentrations (32). The cultures were diluted in half with SD-URA/2% galactose medium and grown overnight (to induce expression from the GAL1 promoter); aliquots were plated on SD-URA medium lacking tryptophan to determine the initial Trp+ mutant frequency and on SD-URA to determine viable cell density. The cultures were diluted to ≈4,000 cells/ml in SD-URA/2% galactose medium with limiting tryptophan (1.5 μM) and divided into 120–240 1-ml cultures, which were incubated at 30°C for 11–14 days. The number of cultures with Trp+ revertants was scored and the mutation rate determined as described (30). Alternatively, cells were pregrown in SD-URA/2% raffinose and then diluted directly to 4,000 cells/ml in SD-URA/2% galactose medium with limiting tryptophan; no difference in spontaneous mutation rates was observed between each growth regime (data not shown). The P0 method for determining mutation rate was not used for strains expressing Mag1 because they produced mutant colonies in every culture; for these strains, the method of the median was used (30). In brief, cultures (5 ml) were grown overnight as before. Cell pellets were washed three times in sterile water and resuspended in SD-URA medium with 2% galactose, and up to 20 cultures (10 ml aliquots containing 3,000 cells) were grown to saturation at 30°C (4–5 days). The cultures were resuspended in 1 ml of sterile water and then eight 0.1-ml aliquots plated on SD-URA medium lacking tryptophan and Trp+ revertants were scored after incubation at 30°C for 3–4 days. The remaining 0.2 ml of each culture was used to determine final viable cell density. Residual growth on plates was determined and the spontaneous mutation rate calculated as described (30). Mutation rates for control strains measured by the P0 method and the method of the median were similar (data not shown).
Determining Spontaneous Mutation Rates for Bacteria.
Bacteria were grown overnight in minimal A medium (33) (supplemented with the appropriate amino acids [(at 40 μg/ml) plus 0.2% maltose and ampicillin (100 μg/ml)] at 37°C to saturation (≈5 × 108 cells/ml). The cultures were diluted 1,000- to 10,000-fold in the same medium also containing 0.2% arabinose to induce expression from the BAD promoter. Fifteen to thirty 1-ml aliquots were distributed, and growth continued at 37°C for 2 days. Pelleted cultures were resuspended in 100 μl of 1X MinA salts (33), and then a portion (10 μl) was diluted appropriately then plated in duplicate on LB plates containing ampicillin (to determine cell viability) whereas the remainder was plated on LB plates containing ampicillin and rifampicin (100 μg/ml). Rifampicin resistant (RifR) colonies were counted after 2 days of incubation at 37°C, and the median mutation frequency was determined (median number of Rif R colonies/mean number of viable cells plated).
Cell Killing.
The MMS sensitivity of yeast strains was evaluated essentially as described (9), with some modifications. In brief, strains were grown in supplemented SD-URA medium (or SD-URA lacking leucine for YEpAPN1-containing strains) containing 2% raffinose or 2% glucose for 2–3 days at 30°C to saturation. To induce expression from the GAL1 promoter, raffinose-grown cultures were resuspended in an equal volume of similar medium containing 2% galactose and grown at 30°C for 3 hr. For glucose repression, cultures were transferred to medium containing 2% glucose. Cultures were exposed to 0.3% MMS for the indicated times, aliquots plated on SD-URA plates containing either 2% galactose or 2% glucose, and colonies scored after 3–4 days incubation at 30°C.
RESULTS
We previously found that the Mag1 DNA glycosylase modestly increases spontaneous mutation in AP endonuclease-deficient yeast (17). Our initial interpretation of these results was that removal of endogenously generated 3MeA or 7-methylguanine produced mutagenic abasic sites. However, subsequent studies revealed that the eukaryotic 3MeA DNA glycosylases have a very broad substrate range, releasing deaminated, oxidized, and alkylated bases (34–37). Indeed, the E. coli Tag enzyme is the only known glycosylase highly specific for 3MeA (38). To determine whether it is the 3MeA repair capability of Mag1 that influences spontaneous mutation, we cloned MAG1 and tag into a regulatable yeast expression plasmid and expressed them in a variety of S. cerevisiae genetic backgrounds. We also cloned the human MAG1 homolog (AAG) into the same expression plasmid to determine whether it too can create a mutator phenotype.
Expression of Mag1, Tag, and AAG in S. cerevisiae.
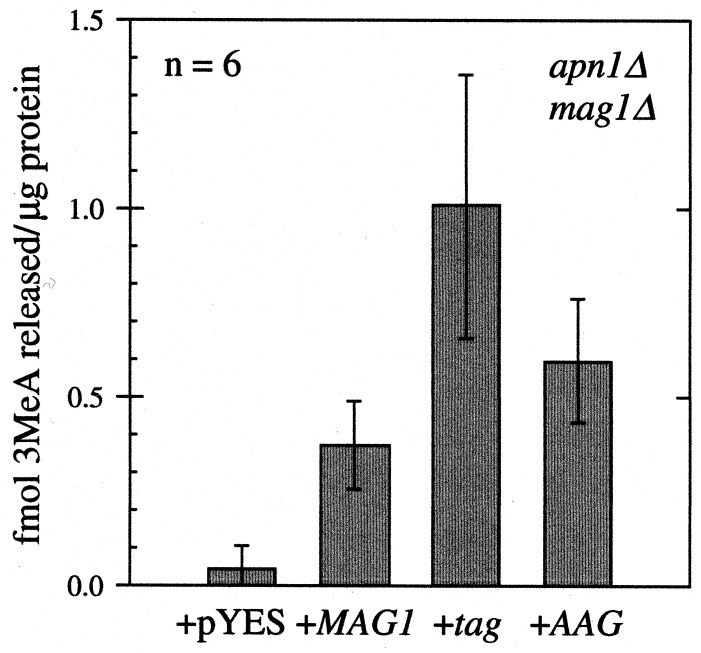
The MAG1-, tag-, and AAG-coding sequences were expressed from the GAL1 promoter in pYES-MAG, pYES-tag, and pYES-AAG. 3MeA DNA glycosylase activity levels were determined after growth under inducing conditions (Fig. 1). Clearly, each construct produced active DNA glycosylase and roughly similar levels were produced from each construct, ranging from a 9- to 24-fold increase above background.
Figure 1.
3MeA DNA glycosylase activities were determined for cell-free extracts after galactose induction for the apn1Δ mag1Δ yeast strain BGY149 containing the control plasmid pYES or expressing MAG1, tag or AAG from plasmids pYES-MAG, pYES-tag or pYES-AAG, expressed as fmol 3MeA released per μg of protein extract. Values reported are the mean ± standard deviation (n = 6).
Mag1 and AAG, but not Tag, Can Elevate Spontaneous Mutation Rates in Yeast.
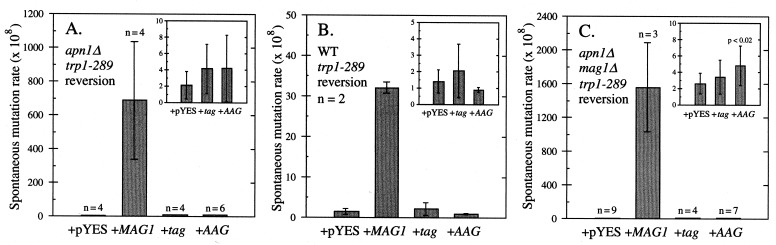
We initially examined the influence of Mag1, Tag, and AAG expression on spontaneous mutation in an AP endonuclease-deficient yeast strain (Fig. 2A). Mag1 expression increased the spontaneous mutation rate relative to the control ≈300-fold, an increase considerably larger than the 4-fold elevation reported previously for the same allele (17). The strong GAL1 promoter (39) likely accounts for this difference (we previously used the relatively weak MAG1 promoter; ref. 17). Given this dramatic 300-fold elevation in mutation rate, we investigated whether Mag1 could elevate spontaneous mutation rates even in the presence of wild-type Apn1 levels. Fig. 2B shows that Mag1 expression elevated the mutation rate ≈20-fold in wild-type cells, an effect not observed previously (17).
Figure 2.
Spontaneous mutation rates determined by fluctuation analysis (or method of the median for strains containing pYES-MAG) after galactose induction for the apn1Δ strain BGY113 (A), the wild-type strain BGY111 (B), and the apn1Δ mag1Δ strain BGY149 (C) of yeast containing the control plasmid pYES or expressing the genes indicated (from plasmids pYES-MAG, pYES-tag, or pYES-AAG). Values reported are the mean ± standard deviation, with the number of independent determinations (n) shown. Note the change in the abscissa scale among A, B, and C. (A, B, and C Inset) Replotted data for pYES, pYES-tag, and pYES-AAG. (C Inset) Spontaneous mutation rate after AAG expression is significantly different (P < 0.02) from the pYES control value, as determined by the Wilcoxon rank-sum test.
No increase in spontaneous mutation was observed when Tag or AAG were expressed in either an AP endonuclease-deficient (Fig. 2A) or -proficient strain (Fig. 2B). However, we suspected that it might be difficult to detect subtle effects of Tag or AAG expression in a Mag+ background. We therefore examined Tag, AAG, and Mag1 expression in a strain deleted for both APN1 and MAG1 (Fig. 2C). Indeed, in this apn1 mag1 double-deletion mutant, there was a small (≈2-fold) but statistically significant elevation in mutation rate for cells expressing human AAG (Fig. 2C Inset); Tag expression had no such effect. Mag1 expression in this background dramatically elevates the mutation rate (≈600-fold, Fig. 2C). We infer that Mag1 and AAG glycosylase each have an activity that Tag lacks and that these activities are responsible for elevating the spontaneous mutation rate. It should be noted that the mutation rates determined here reflect reversion at trp1–289a; because this is an amber suppressible allele, we are probably monitoring more than a single mutational event (17).
Elevation of Spontaneous Mutation in Bacteria by Mag1, but not Tag.
The fact that Mag1 appears to be a stronger mutator than AAG cannot be attributed to pYES-MAG expressing more glycosylase activity than pYES-AAG, at least as measured by 3MeA release (Fig. 1). However, Mag1 could be more active in its native yeast environment than are Tag and AAG, perhaps because of efficient nuclear localization or interaction with essential cofactors. To test this possibility, we expressed Mag1 and Tag in AP endonuclease-deficient E. coli, reasoning that Mag1 would no longer have yeast-specific advantage(s) and that Tag would be in its native environment. However, the results exactly paralleled those obtained in yeast. Tag failed to elevate mutation to rifampicin resistance in E. coli, but Mag1 elevated such mutation by an average of ≈200-fold, despite similar Mag1 and Tag 3MeA DNA glycosylase levels relative to the control strain (Table 2). We infer that the dramatic mutator effect observed for Mag1 is caused by intrinsic properties of this enzyme. In addition, the apparent plating efficiency of strain BW528 containing p24MAG induced with arabinose was reduced by one to three orders of magnitude. No such decrease in plating efficiency was observed after induction of cells containing pBAD24 or p24Tag (data not shown).
Table 2.
Median mutation frequencies in bacteria
| Experiment no. | Strain BW528 | No. of cultures | Median RifR mutation Frequency, × 108 | Fold increase* | Fold increase* in 3MeA DNA glycosylase activity† |
|---|---|---|---|---|---|
| 1 | +pBAD24 | 26 | 1.5 | 1 | 1 |
| +p24Tag | 19 | 1.4 | 0.9 | 12.7 ± 3.7 | |
| +p24MAG | 29 | 133 | 89 | 6.7 ± 1.6 | |
| 2 | +pBAD24 | 15 | 13.2 | 1 | |
| +p24Tag | 15 | 14.0 | 1.1 | ||
| +p24MAG | 15 | 618 | 47 | ||
| 3 | +pBAD24 | 30 | 0.7 | 1 | |
| +p24Tag | 30 | 0.3 | 0.4 | ||
| +p24MAG | 30 | 333 | 476 |
Relative to pBAD24 control.
Determined from similar cultures as described in Materials and Methods (mean ± SD, n = 3).
Apn1 Suppresses the Mag1-Induced Increase in Spontaneous Mutation in Yeast.
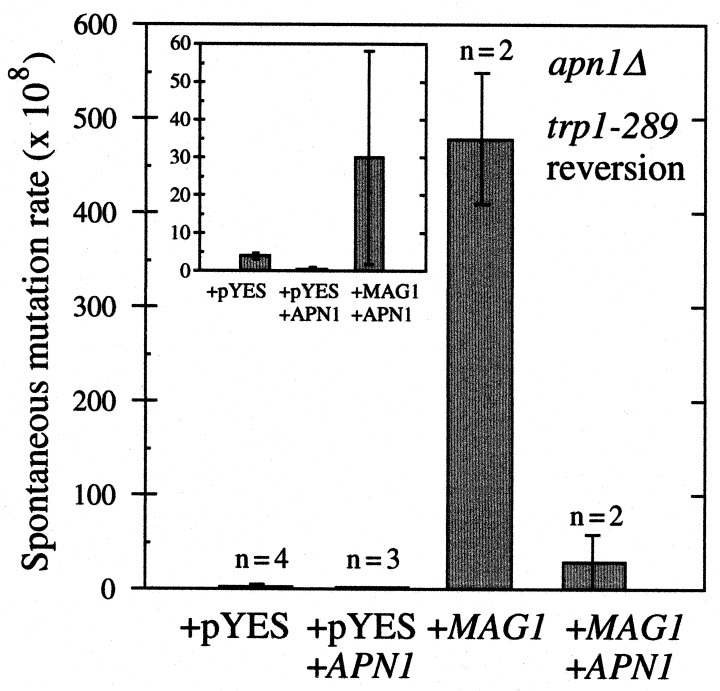
We reasoned that if Mag1-generated abasic sites are responsible for the Mag1 mutator phenotype, coexpression of Apn1 should suppress this phenotype. Fig. 3 confirms that Apn1 expression dramatically suppresses the elevation in spontaneous mutation caused by Mag1. This suppression was not caused by decreased Mag1 activity in the presence of the YEpAPN1 plasmid (data not shown). The observed suppression by Apn1 of the majority of the Mag1-induced increase in spontaneous mutation is presumably caused by Apn1 initiating the repair of glycosylase-induced abasic sites. These data argue that the abasic sites generated by Mag1 are responsible for the mutator phenotype and that the subsequent steps of base excision repair are not limiting.
Figure 3.
Spontaneous mutation rates determined by the method of the median after galactose induction of the apn1Δ strain BGY113 containing plasmid pYES, pYES plus YEpAPN1 (which expresses APN1), pYES-MAG, or pYES-MAG plus YEpAPN1. The values reported are the mean ± standard deviation. (Inset) Replotted data for pYES, pYES plus YEpAPN1, and pYES-MAG plus YEpAPN1.
Rev1, Rev3, and Rev7 Are Required for the Mag1-Induced Mutator Phenotype.
The REV1, REV3, and REV7 genes are members of the RAD6 postreplicative/error-prone DNA repair epistasis group in S. cerevisiae, and their proteins are required for UV-induced mutability (2, 40). In vitro biochemical studies have demonstrated that the REV1 deoxycytidyl transferase, in conjunction with the REV3/REV7 DNA polymerase ζ, can perform bypass DNA synthesis opposite abasic sites (19, 20). We examined whether the Mag1-induced mutator phenotype depends on these three gene products. Indeed, the Mag1-induced elevation in Trp+ reversion rate is virtually eliminated in strains deleted for the REV1, REV3, or REV7 gene (+pYES-MAG, Table 3). In addition, the apparent plating efficiency of cells containing pYES-MAG after galactose induction was decreased ≈10-fold relative to the control strain (+pYES) or relative to the uninduced (glucose-grown, +pYES-MAG) controls (data not shown). Consistent with previous suggestions (41–43), the production of spontaneous Trp+ revertants in the absence of Mag1 expression also appears to be suppressed in the rev-deletion backgrounds (+pYES, Table 3). Importantly, the rev-deletion strains do produce occasional Trp+ revertants spontaneously and after mutagen treatment (data not shown). These results, together with those presented above, suggest that most, if not all, of the Mag1-induced mutations are fixed by a process requiring Rev1/Rev3/Rev7-catalyzed lesion bypass DNA synthesis at abasic sites.
Table 3.
Spontaneous mutation rates in yeast
| Strain | Relevant genotype | No. Trp+ revertants/cell/generation (× 108)
|
|
|---|---|---|---|
| +pYES | +pYES-MAG | ||
| BGY149 | apn1Δ mag1Δ | 2.6 ± 1.3 | 1,563 ± 532 |
| BGY167 | apn1Δ mag1Δ rev1Δ | 0.88 | 8.1 ± 0.9 |
| BGY168 | apn1Δ mag1Δ rev3Δ | 1.1 | 0.63 ± 0.75 |
| BGY169 | apn1Δ mag1Δ rev7Δ | 0.80 | 0.50 ± 0.17 |
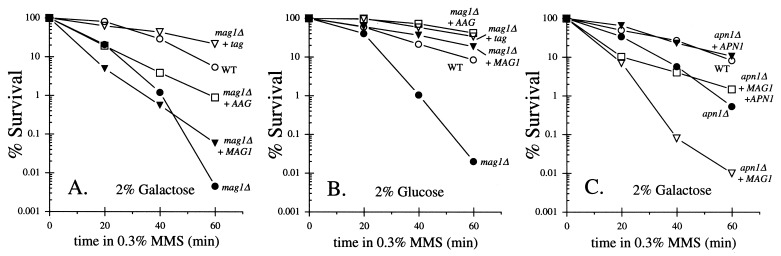
Mag1 and AAG, but not Tag, Sensitize Yeast to MMS.
The pYES-MAG, pYES-tag, and pYES-AAG plasmids were checked for their ability to suppress the alkylation sensitivity of a yeast mag1-deletion strain. Unexpectedly, after galactose induction, only Tag expression rescued this strain from killing by MMS; AAG gave only partial protection, whereas Mag1 gave no protection whatsoever (Fig. 4A). High level expression of 3MeA DNA glycosylases was previously shown to sensitize bacterial and mammalian cells to alkylating agents (44, 45). When cells were grown in glucose, which permits at best only low level expression from the GAL1 promoter (46, 47), all three glycosylases fully complemented the mag1-deletion defect (Fig. 4B). We infer the following: (i) all three constructs produce functional 3MeA DNA glycosylases (in agreement with data from Fig. 1) and (ii) high levels of Mag1 and AAG sensitize yeast to MMS. Furthermore, for Mag1 expression, the sensitization is significantly suppressed by Apn1 coexpression (Fig. 4C), suggesting that the increased MMS cytotoxicity is associated with the generation of abasic sites (coexpression of Apn1 with AAG was not tested).
Figure 4.
MMS killing after induction of the GAL1 promoter with galactose (A) or repression with glucose (B) for the Mag+ wild-type yeast strain BGY111 containing the control plasmid pYES (○) or its isogenic mag1Δ derivative strain BGY148 containing the plasmid pYES (•), pYES-tag (▿), pYES-MAG (▾) or pYES-AAG (□). A representative experiment is shown in each case; the relative survival between the various strains was always consistent. In addition, for the galactose-induced cells, qualitatively similar results were obtained in apn1Δ (BGY113) and apn1Δ mag1Δ (BGY149) strain backgrounds (data not shown). (C) MMS killing after galactose induction for the Mag+ wild-type strain BGY111 containing the control plasmid pYES (○) or its isogenic apn1Δ derivative strain BGY113 containing the plasmids pYES(•), pYES-MAG (▿), YEpAPN1 (▾), or pYES-MAG and YEpAPN1 (□).
DISCUSSION
The spontaneous mutation rate, expressed per genome per replication, is remarkably constant over a wide range of organisms and genome sizes (48). The spontaneous mutation rate probably reflects a balance between the chemical and biological processes that generate mutations and the biological processes that suppress them. Intrinsic to this idea is the potential for this balance to be upset, thus generating antimutator or mutator phenotypes. Here, we describe a mechanism for producing a strong mutator phenotype in S. cerevisiae.
The majority of spontaneous mutations in wild-type S. cerevisiae are thought to be generated by the mutagenic repair of spontaneously produced DNA lesions (17, 18, 41, 42, 49). This mutagenic repair is part of the complex and poorly understood RAD6 pathway that includes the REV genes whose products are required for the replication bypass of DNA lesions produced by both endogenous and exogenous agents (2, 40). REV1 was shown recently to encode a deoxycytidyl transferase that preferentially inserts cytidine opposite an abasic site in vitro; extension from this cytidine residue “paired” with an abasic site requires DNA polymerase ζ, composed of two subunits encoded by REV3 and REV7 (19, 20). This model predicts that spontaneous mutations resulting from endogenous abasic sites could be suppressed by blocking Rev1/Rev3/Rev7-dependent translesion bypass. Our results are consistent with this model. The elevation in Trp+ reversion rates produced by expression of the Mag1 3MeA DNA glycosylase was almost completely suppressed in rev1-, rev3- or rev7-deletion mutant backgrounds. The requirement of the Rev1 deoxycytidyl transferase for the Mag1 mutator effect predicts a preponderance of base substitutions by cytosine in the mutational spectrum and this is under active study. It also predicts that the Mag1 target lesions responsible for the mutator phenotype are unlikely to be guanine lesions.
In E. coli, a number of genes, when defective, produce spontaneous mutation rates orders of magnitude higher than wild-type rates (3). For example, the strongest known E. coli mutator alleles map to dnaQ, which encodes the ɛ proofreading subunit of the DNA polymerase III holoenzyme (50, 51). Such mutations elevate spontaneous mutation frequencies 10,000- to 100,000-fold (52, 53). In S. cerevisiae, most mutator phenotypes have elevations in mutation rates of ≈50-fold (2). Even defects in proofreading DNA polymerases do not elevate mutation to the levels observed in E. coli. DNA polymerase δ mutations increase spontaneous mutation rates ≈100- to 600-fold (54, 55); such strong mutator phenotypes also are associated with defects in DNA mismatch repair (54, 56–58). Thus, the Mag1-induced 600-fold elevation in the S. cerevisiae spontaneous mutation rate reported here is among the strongest mutator phenotypes observed in yeast.
Evidence supports a multistep model for human tumor formation involving several genetic changes. However, current estimates of human spontaneous mutation rates cannot support the accumulation of this number of genetic changes in a single cell (4). Thus, a necessary and early event in carcinogenesis may be the generation of a mutator phenotype (7, 59). Defects in DNA mismatch repair generate a mutator phenotype in human cells (60) and are a risk factor for colorectal and other cancers (61, 62). As a result, there is considerable interest in identifying mutations in other DNA repair genes that increase spontaneous mutation, and thus have the potential to increase cancer risk. The data presented here highlight a mechanism by which spontaneous mutation rates may be modulated. An imbalance between AP endonuclease and one particular DNA glycosylase produced a striking mutator phenotype in yeast. This situation is distinct from, but analogous to, imbalanced dNTP levels and methylation/mismatch repair imbalances; both of these conditions have been observed to lead to mutator phenotypes (63–65). Glycosylase to AP endonuclease ratio imbalances could arise by promoter mutations that affect DNA repair gene expression, by mutations that alter the Km or kCAT of either enzyme, by mutations that modulate nuclear localization, and so on. Imbalances in base excision repair pathway enzymes may represent a hitherto unrecognized source of increased cancer risk.
We have shown that high levels of the Mag1 and AAG glycosylases sensitize yeast cells to the cytotoxic effects of MMS, an effect reported previously for alkA expression in E. coli (45) and AAG expression in Chinese hamster cells (44). Coquerelle et al. (44) also reported that AAG overexpression increased sensitivity to MMS-induced chromosome damage and inhibition of DNA replication. These results suggest that base excision repair imbalances influence how cells respond to exogenous agents, whereas our results show that such imbalances also can influence cellular responses to endogenous agents.
The chemical nature of the DNA lesion(s) that Mag1 and AAG act on to produce a mutator phenotype remains to be determined. However, the lesion is unlikely to be 3MeA because robust expression of Tag, which is highly specific for 3MeA (5, 66), fails to generate a mutator phenotype. Eukaryotic 3MeA DNA glycosylases were reported to have a modest ability to release potentially mutagenic 8-oxoguanine lesions from DNA (67). However, 8-oxoguanine removal also is unlikely to be the lesion responsible for the mutator activity because the Mag1 glycosylase appears to have no detectable 8-oxoguanine-specific activity (data not shown).
Mag1 and AAG (but not Tag) can remove ethenoadenine and hypoxanthine from DNA, but AAG is 200- to 300-fold more efficient than Mag1 for both of these base lesions (12, 13). Because Mag1 is a much stronger mutator than AAG in this yeast system, the release of hypoxanthine or ethenoadenine is unlikely to account for their mutator activity. It also is possible that, in vivo, Mag1 and AAG (but not Tag) are removing normal bases from DNA. Indeed, it was reported recently that purified Mag1 and AAG, but not Tag, display a very modest but significant ability to release guanine from DNA in vitro (68). Moreover, others have shown that mutant forms of the human uracil DNA glycosylase can remove normal pyrimidines and can produce a modest mutator phenotype in E. coli (69). These results raise the possibility that subtle changes in the human AAG DNA repair enzyme could modify its substrate specificity to include normal bases or common base lesions, thus increasing its ability to act as a mutator glycosylase.
Acknowledgments
We thank Alexander Long and Tom Ellenberger (Harvard University) for sequencing the tag-coding sequence in pYES-tag and David J. Hunter (Harvard School of Public Health) for statistical advice. We also would like to thank one of the reviewers for an extremely thorough and helpful review. This work was supported by a National Institute on Environmental Health Sciences grant (P01-ES03926) and a National Cancer Institute grant (R01-CA5502). B.J.G. was supported by an National Research Service Award Training grant (5T32CA09078-19). L.M.P. was supported by a Pharmaceutical and Research Manufacturers of America Foundation Advanced Predoctoral Fellowship in Toxicology. L.D.S. is a Burroughs Wellcome Toxicology Scholar.
ABBREVIATIONS
- AP
apurinic/apyrimidinic
- 3MeA
3-methyladenine
- MMS
methyl methanesulfonate
- RifR
rifampicin resistant
- pYES
pYES2.0
- SD
synthetic dextrose minimal medium
- SD-URA
SD lacking uracil
References
- 1.Friedberg E C, Walker G C, Siede W. DNA Repair and Mutagenesis. Washington, DC: Am. Soc. Microbiol.; 1995. [Google Scholar]
- 2.Lawrence C W. Adv Genet. 1982;21:173–254. doi: 10.1016/s0065-2660(08)60299-0. [DOI] [PubMed] [Google Scholar]
- 3.Miller J H. Annu Rev Microbiol. 1996;50:625–643. doi: 10.1146/annurev.micro.50.1.625. [DOI] [PubMed] [Google Scholar]
- 4.Hall M, Johnson R T. Mol Aspects Med. 1996;17:235–383. doi: 10.1016/s0098-2997(96)00001-5. [DOI] [PubMed] [Google Scholar]
- 5.Wilson D M, Engelward B P, Samson L. In: DNA Damage and Repair: Biochemistry, Genetics and Cell Biology. Nickoloff J A, Hoekstra M F, editors. Vol. 1. Totowa, NJ: Humana Press; 1997. [Google Scholar]
- 6.Wood R D. Annu Rev Biochem. 1996;65:135–167. doi: 10.1146/annurev.bi.65.070196.001031. [DOI] [PubMed] [Google Scholar]
- 7.Loeb L A. Annu Rev Genet. 1986;20:201–230. doi: 10.1146/annurev.ge.20.120186.001221. [DOI] [PubMed] [Google Scholar]
- 8.Strauss B S. BioEssays. 1991;13:79–84. doi: 10.1002/bies.950130206. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Derfler B, Maskati A, Samson L. Proc Natl Acad Sci USA. 1989;86:7961–7965. doi: 10.1073/pnas.86.20.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjoras M, Klungland A, Johansen R F, Seeberg E. Biochemistry. 1995;34:4577–4582. doi: 10.1021/bi00014a010. [DOI] [PubMed] [Google Scholar]
- 11.Matijasevic Z, Boosalis M, MacKay W, Samson L. Proc Natl Acad Sci USA. 1993;90:11855–11859. doi: 10.1073/pnas.90.24.11855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saparbaev M, Kleibl K, Laval J. Nucleic Acids Res. 1995;23:3750–3755. doi: 10.1093/nar/23.18.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saparbaev M, Laval J. Proc Natl Acad Sci USA. 1994;91:5873–5877. doi: 10.1073/pnas.91.13.5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson A W, Demple B. J Biol Chem. 1988;263:18009–18016. [PubMed] [Google Scholar]
- 15.Johnson A W, Demple B. J Biol Chem. 1988;263:18017–18022. [PubMed] [Google Scholar]
- 16.Ramotar D, Popoff S C, Gralla E B, Demple B. Mol Cell Biol. 1991;11:4537–4544. doi: 10.1128/mcb.11.9.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao W, Samson L. Proc Natl Acad Sci USA. 1993;90:2117–2121. doi: 10.1073/pnas.90.6.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunz B A, Henson E S, Roche H, Ramotar D, Nunoshiba T, Demple B. Proc Natl Acad Sci USA. 1994;91:8165–8169. doi: 10.1073/pnas.91.17.8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson J R, Lawrence C W, Hinkle D C. Nature (London) 1996;382:729–731. doi: 10.1038/382729a0. [DOI] [PubMed] [Google Scholar]
- 20.Nelson J R, Lawrence C W, Hinkle D C. Science. 1996;272:1646–1649. doi: 10.1126/science.272.5268.1646. [DOI] [PubMed] [Google Scholar]
- 21.Guzman L-M, Belin D, Carson M J, Beckwith J. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen B J, Carroll P, Samson L. J Bacteriol. 1994;176:6255–6261. doi: 10.1128/jb.176.20.6255-6261.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Popoff S C, Spira A I, Johnson A W, Demple B. Proc Natl Acad Sci USA. 1990;87:4193–4197. doi: 10.1073/pnas.87.11.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 25.Alani E, Cao L, Kleckner N. Genetics. 1987;116:541–545. doi: 10.1534/genetics.112.541.test. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemontt J F. Genetics. 1971;68:21–33. doi: 10.1093/genetics/68.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boeke J D, Trueheart J, Natsoulis G, Fink G R. Methods Enzymol. 1987;154:164–175. doi: 10.1016/0076-6879(87)54076-9. [DOI] [PubMed] [Google Scholar]
- 28.Cunningham R P, Saporito S M, Spitzer S G, Weiss B. J Bacteriol. 1986;168:1120–1127. doi: 10.1128/jb.168.3.1120-1127.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. In: Current Protocols in Molecular Biology. Janssen K, editor. Vol. 2. New York: Wiley; 1994. [Google Scholar]
- 30.von Borstel R C. Methods Cell Biol. 1978;20:1–24. doi: 10.1016/s0091-679x(08)62005-1. [DOI] [PubMed] [Google Scholar]
- 31.Luria S E, Delbruck M. Genetics. 1943;28:491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rose M D, Winston F, Hieter P. Methods in Yeast Genetics: A Laboratory Course Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1990. [Google Scholar]
- 33.Miller J H. A Short Course in Bacterial Genetics. Plainview, NY: Cold Spring Harbor Lab. Press; 1992. [Google Scholar]
- 34.Hickson I D. Molecular Biology Intelligence Unit. New York: Chapman & Hall; 1997. [Google Scholar]
- 35.Memisoglu A, Samson L. Crit Rev Biochem Mol Biol. 1996;31:405–447. doi: 10.3109/10409239609108724. [DOI] [PubMed] [Google Scholar]
- 36.Seeberg E, Eide L, Bjoras M. Trends Biochem Sci. 1995;20:391–397. doi: 10.1016/s0968-0004(00)89086-6. [DOI] [PubMed] [Google Scholar]
- 37.Singer B, Hang B. Chem Res Toxicol. 1997;10:713–732. doi: 10.1021/tx970011e. [DOI] [PubMed] [Google Scholar]
- 38.Bjelland S, Seeberg E. Nucleic Acids Res. 1987;15:2787–2801. doi: 10.1093/nar/15.7.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnston M, Davis R W. Mol Cell Biol. 1984;4:1440–1448. doi: 10.1128/mcb.4.8.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lawrence C W. BioEssays. 1994;16:253–258. doi: 10.1002/bies.950160408. [DOI] [PubMed] [Google Scholar]
- 41.Quah S-K, von Borstel R C, Hastings P J. Genetics. 1980;96:819–839. doi: 10.1093/genetics/96.4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roche H, Gietz R D, Kunz B A. Genetics. 1994;137:637–646. doi: 10.1093/genetics/137.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tuite M F, Cox B S. Genetics. 1980;95:611–630. doi: 10.1093/genetics/95.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coquerelle T, Dosch J, Kaina B. Mutat Res. 1995;336:9–17. doi: 10.1016/0921-8777(94)00035-5. [DOI] [PubMed] [Google Scholar]
- 45.Kaasen I, Evensen G, Seeberg E. J Bacteriol. 1986;168:642–647. doi: 10.1128/jb.168.2.642-647.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnston M. Microbiol Rev. 1987;51:458–476. doi: 10.1128/mr.51.4.458-476.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao W, Singh K K, Chen B, Samson L. Mol Cell Biol. 1993;13:7213–7221. doi: 10.1128/mcb.13.12.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drake J W, Charlesworth B, Charlesworth D, Crow J F. Genetics. 1998;148:1667–1686. doi: 10.1093/genetics/148.4.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hastings P J, Quah S-K, von Borstel R C. Nature (London) 1976;264:719–722. doi: 10.1038/264719a0. [DOI] [PubMed] [Google Scholar]
- 50.Scheuermann R, Echols H. Proc Natl Acad Sci USA. 1984;81:7747–7751. doi: 10.1073/pnas.81.24.7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scheuermann R, Tam S, Burgers P M J, Lu C, Echols H. Proc Natl Acad Sci USA. 1983;80:7085–7989. doi: 10.1073/pnas.80.23.7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cox E C, Horner D L. Genetics. 1982;100:7–18. doi: 10.1093/genetics/100.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Degnen G E, Cox E C. J Bacteriol. 1974;117:477–487. doi: 10.1128/jb.117.2.477-487.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morrison A, Johnson A L, Johnston L H, Sugino A. EMBO J. 1993;12:1467–1473. doi: 10.1002/j.1460-2075.1993.tb05790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simon M, Giot L, Faye G. EMBO J. 1991;10:2165–2170. doi: 10.1002/j.1460-2075.1991.tb07751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morrison A, Sugino A. Mol Gen Genet. 1994;242:289–296. doi: 10.1007/BF00280418. [DOI] [PubMed] [Google Scholar]
- 57.Tishkoff D X, Filosi N, Gaida G M, Kolodner R. Cell. 1997;88:253–263. doi: 10.1016/s0092-8674(00)81846-2. [DOI] [PubMed] [Google Scholar]
- 58.Tran H T, Keen J D, Kricker M, Resnick M A, Gordenin D A. Mol Cell Biol. 1997;17:2859–2865. doi: 10.1128/mcb.17.5.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nowell P C. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 60.Glaab W E, Tindall K R. Carcinogenesis. 1997;18:1–8. doi: 10.1093/carcin/18.1.1. [DOI] [PubMed] [Google Scholar]
- 61.Kolodner R D, Alani E. Curr Opin Biotechnol. 1994;5:585–594. doi: 10.1016/0958-1669(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 62.Peltomaki P, de la Chapelle A. Adv Cancer Res. 1997;71:93–119. doi: 10.1016/s0065-230x(08)60097-4. [DOI] [PubMed] [Google Scholar]
- 63.Kohalmi S E, Glattke M, McIntosh E M, Kunz B A. J Mol Biol. 1991;220:933–946. doi: 10.1016/0022-2836(91)90364-c. [DOI] [PubMed] [Google Scholar]
- 64.Marinus M G, Morris N R. Mutat Res. 1975;28:15–26. doi: 10.1016/0027-5107(75)90309-7. [DOI] [PubMed] [Google Scholar]
- 65.Marinus M G, Poteete A, Arraj J A. Gene. 1984;28:123–125. doi: 10.1016/0378-1119(84)90095-7. [DOI] [PubMed] [Google Scholar]
- 66.Krokan H E, Standal R, Slupphaug G. Biochem J. 1997;325:1–16. doi: 10.1042/bj3250001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bessho T, Roy R, Yamamoto K, Kasai H, Nishimura S, Tano K, Mitra S. Proc Natl Acad Sci USA. 1993;90:8901–8904. doi: 10.1073/pnas.90.19.8901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berdal K G, Johansen R F, Seeberg E. EMBO J. 1998;17:363–367. doi: 10.1093/emboj/17.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kavli B, Slupphaug G, Mol C D, Arvai A S, Petersen S B, Tainer J A, Krokan H E. EMBO J. 1996;15:3442–3447. [PMC free article] [PubMed] [Google Scholar]