Abstract
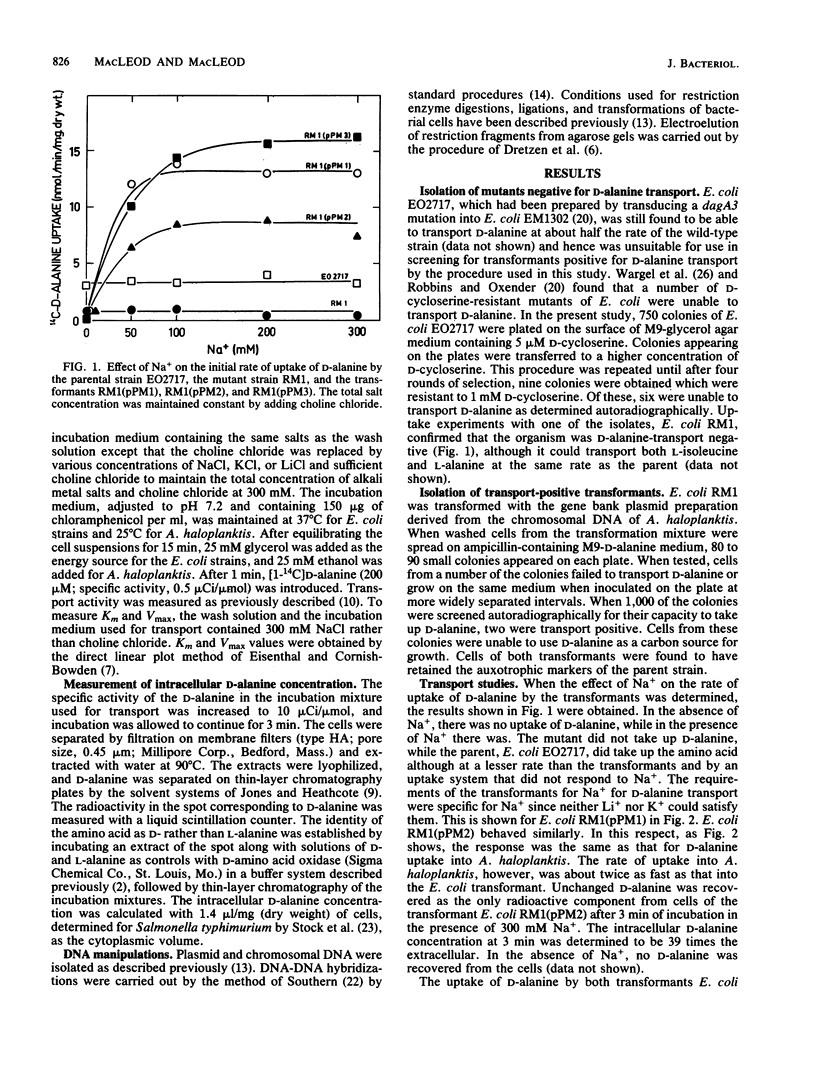
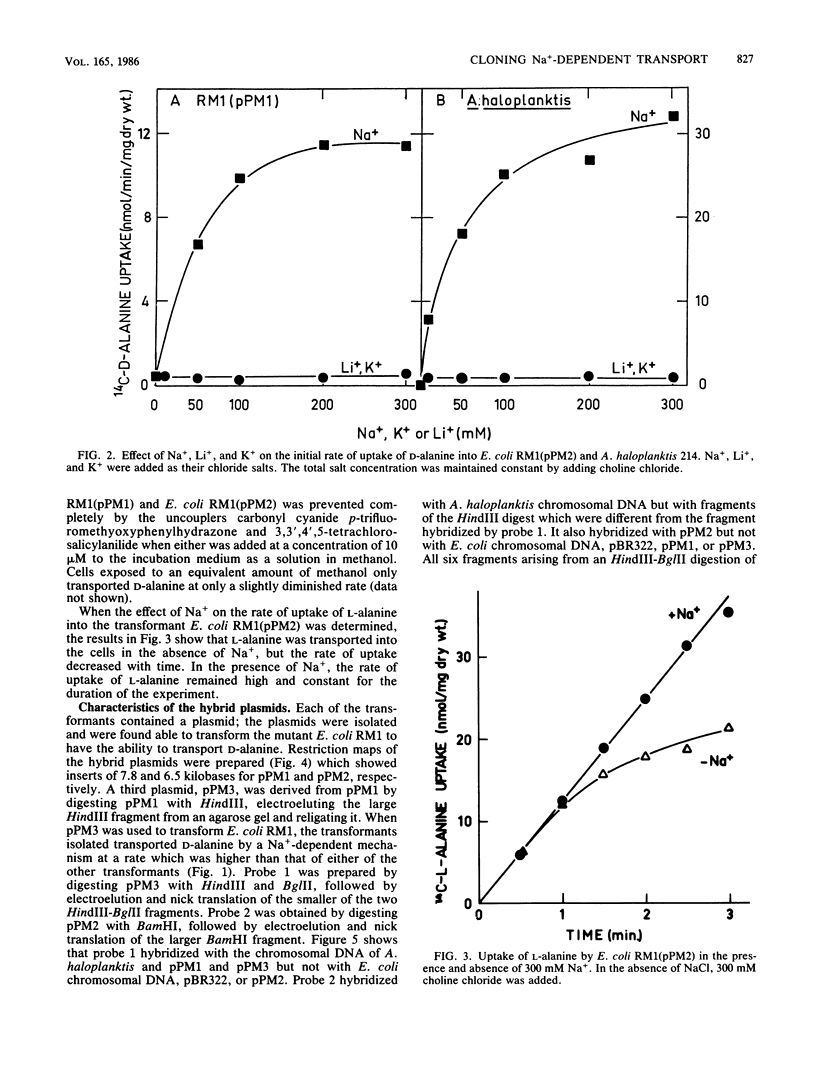
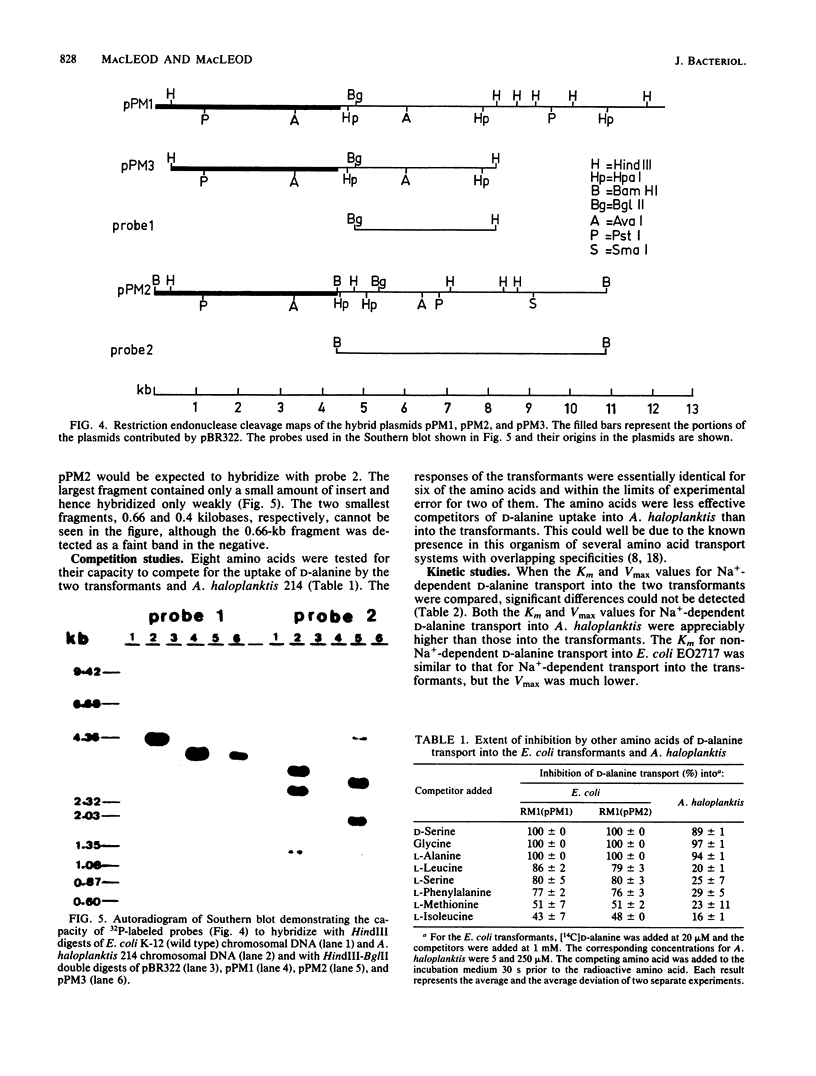
The transport of D-alanine by Escherichia coli K-12 neither requires nor is stimulated by Na+. The transport of D-alanine by the marine bacterium Alteromonas haloplanktis 214 requires Na+ specifically. Mutants of E. coli which were unable to transport D-alanine were isolated by enrichment for D-cycloserine resistance. One of the mutants was transformed with a gene bank of A. haloplanktis chromosomal DNA. Two transformants, E. coli RM1(pPM1) and E. coli RM1(pPM2) were able to transport D-alanine by a Na+-dependent mechanism. Li+ and K+ were unable to replace Na+. Both transformants contained chimeric plasmids with inserts which hybridized with A. haloplanktis but not E. coli chromosomal DNA or each other. Despite the lack of homology between the inserts, Na+-dependent D-alanine transport in the two transformants could not be distinguished either by kinetic studies or by differences in the capacity of various amino acids to compete for D-alanine uptake.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger E. A., Heppel L. A. Different mechanisms of energy coupling for the shock-sensitive and shock-resistant amino acid permeases of Escherichia coli. J Biol Chem. 1974 Dec 25;249(24):7747–7755. [PubMed] [Google Scholar]
- Cosloy S. D. D-serine transport system in Escherichia coli K-12. J Bacteriol. 1973 May;114(2):679–684. doi: 10.1128/jb.114.2.679-684.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Charamella L. J., Berg C. M., Harris P. E. Kinetic and genetic analyses of D-cycloserine inhibition and resistance in Escherichia coli. J Bacteriol. 1965 Nov;90(5):1238–1250. doi: 10.1128/jb.90.5.1238-1250.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein J. E., MacLeod R. A. Characterization of neutral amino acid transport in a marine pseudomonad. J Bacteriol. 1975 Dec;124(3):1177–1190. doi: 10.1128/jb.124.3.1177-1190.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K., Heathcote J. G. The rapid resolution of naturally occurring amino acids by thin-layer chromatography. J Chromatogr. 1966 Sep;24(1):106–111. doi: 10.1016/s0021-9673(01)98107-5. [DOI] [PubMed] [Google Scholar]
- Khanna G., DeVoe L., Brown L., Niven D. F., MacLeod R. A. Relationship between ion requirements for respiration and membrane transport in a marine bacterium. J Bacteriol. 1984 Jan;157(1):59–63. doi: 10.1128/jb.157.1.59-63.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K. The role of Na+ in transport processes of bacterial membranes. Biochim Biophys Acta. 1979 Dec 20;559(4):377–397. doi: 10.1016/0304-4157(79)90011-x. [DOI] [PubMed] [Google Scholar]
- MacLeod R. A., Hadley R. G., Szalay A. A., Vink B., MacLeod P. R. Expression of genes from the marine bacterium Alteromonas haloplanktis 214 in Escherichia coli K-12. Arch Microbiol. 1985 Aug;142(3):248–252. doi: 10.1007/BF00693398. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Niven D. F., MacLeod R. A. Sodium ion-proton antiport in a marine bacterium. J Bacteriol. 1978 Jun;134(3):737–743. doi: 10.1128/jb.134.3.737-743.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niven D. F., MacLeod R. A. Sodium ion-substrate symport in a marine bacterium. J Bacteriol. 1980 May;142(2):603–607. doi: 10.1128/jb.142.2.603-607.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce S. M., Hildebrandt V. A., Lee T. Third system for neutral amino acid transport in a marine pseudomonad. J Bacteriol. 1977 Apr;130(1):37–47. doi: 10.1128/jb.130.1.37-47.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. C., Oxender D. L. Transport systems for alanine, serine, and glycine in Escherichia coli K-12. J Bacteriol. 1973 Oct;116(1):12–18. doi: 10.1128/jb.116.1.12-18.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. R. Mapping of a D-cycloserine resistance locus in escherichia coli K-12. J Bacteriol. 1972 Aug;111(2):622–624. doi: 10.1128/jb.111.2.622-624.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Rauch B., Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J Biol Chem. 1977 Nov 10;252(21):7850–7861. [PubMed] [Google Scholar]
- Tatum E. L., Lederberg J. Gene Recombination in the Bacterium Escherichia coli. J Bacteriol. 1947 Jun;53(6):673–684. doi: 10.1128/jb.53.6.673-684.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya T., Ottina K., Moriyama Y., Newman M. J., Wilson T. H. Solubilization and reconstitution of the melibiose carrier from a plasmid-carrying strain of Escherichia coli. J Biol Chem. 1982 May 10;257(9):5125–5128. [PubMed] [Google Scholar]
- Wargel R. J., Hadur C. A., Neuhaus F. C. Mechanism of D-cycloserine action: transport mutants for D-alanine, D-cycloserine, and glycine. J Bacteriol. 1971 Mar;105(3):1028–1035. doi: 10.1128/jb.105.3.1028-1035.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazyu H., Shiota-Niiya S., Shimamoto T., Kanazawa H., Futai M., Tsuchiya T. Nucleotide sequence of the melB gene and characteristics of deduced amino acid sequence of the melibiose carrier in Escherichia coli. J Biol Chem. 1984 Apr 10;259(7):4320–4326. [PubMed] [Google Scholar]