Abstract
Secretion vectors based on the genes from Bacillus amyloliquefaciens P for alkaline protease (aprBamP) and neutral protease (nprBamP) were constructed. With both aprBamP and nprBamP, a unique restriction site was introduced 3' of the predicted signal coding region by using the technique of oligonucleotide-directed mutagenesis. The new sites enabled us to fuse a heterologous gene to the expression and secretion elements. We used the protein A gene (spa) from Staphylococcus aureus as a heterologous gene. Bacillus subtilis cells carrying the resulting apr-spa or npr-spa gene fusions synthesized the fusion protein. B. subtilis cells were also capable of removing the signal peptide from the fusion protein, as indicated by the appearance of processed protein A into the growth medium. In addition, these gene fusions allowed us to identify the signal processing site of both the APR-SPA and NPR-SPA proteins.
Full text
PDF

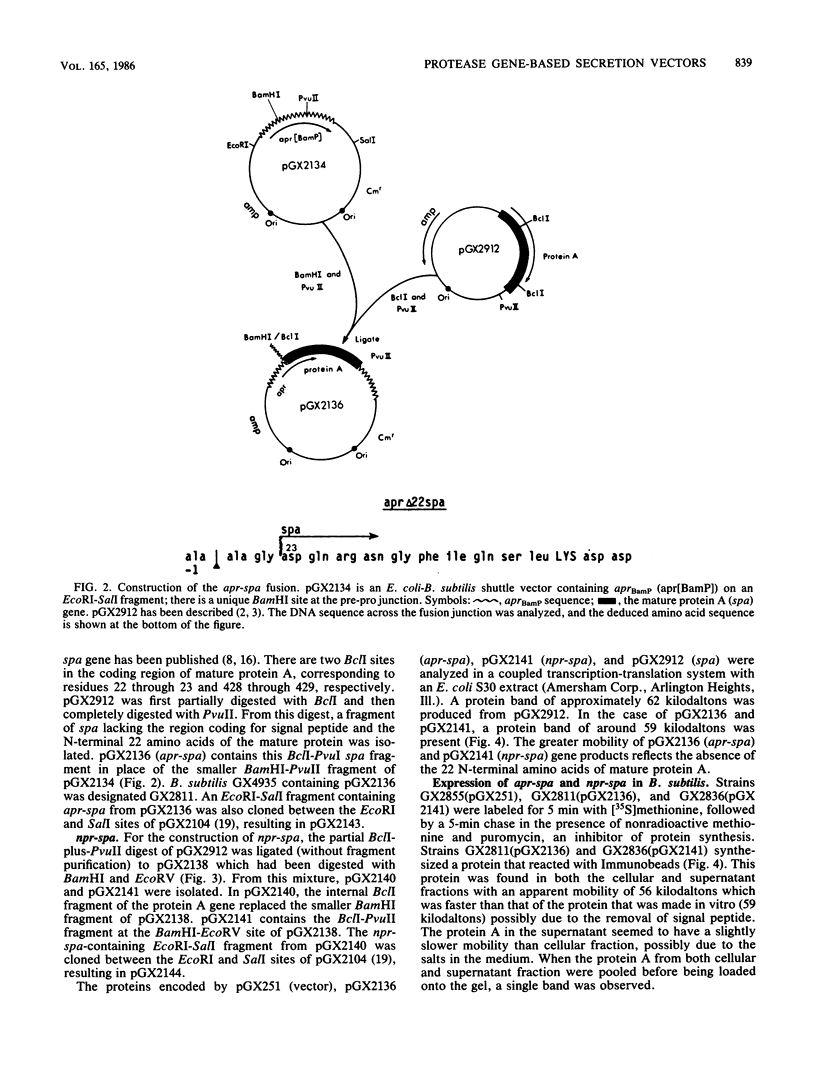
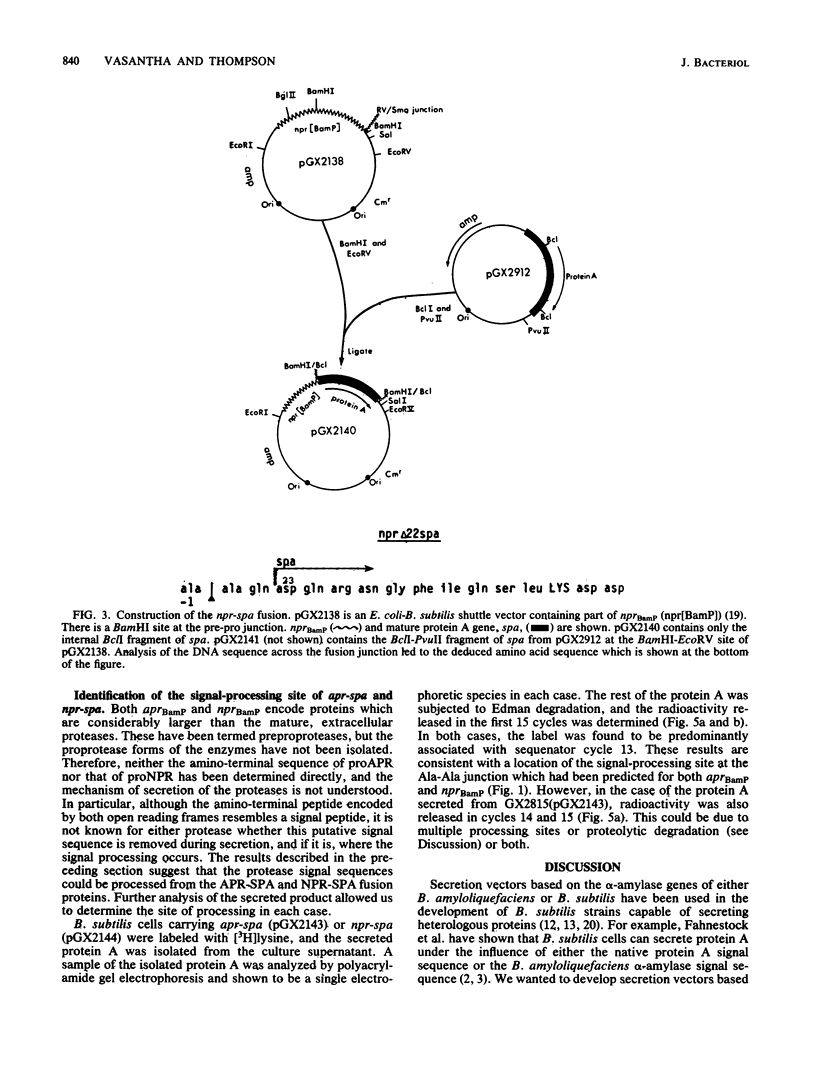
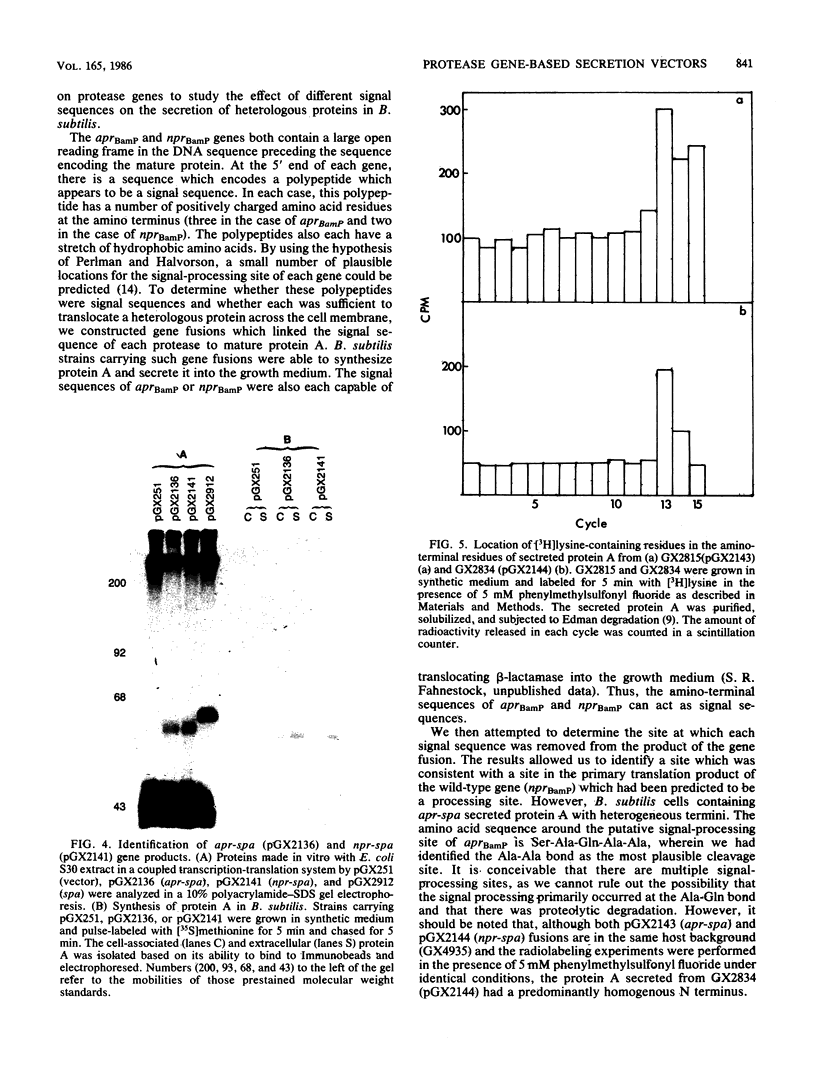

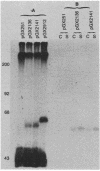
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahnestock S. R., Fisher K. E. Expression of the staphylococcal protein A gene in Bacillus subtilis by gene fusions utilizing the promoter from a Bacillus amyloliquefaciens alpha-amylase gene. J Bacteriol. 1986 Mar;165(3):796–804. doi: 10.1128/jb.165.3.796-804.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould A. R., May B. K., Elliott W. H. Release of extracellular enzymes from Bacillus amyloliquefaciens. J Bacteriol. 1975 Apr;122(1):34–40. doi: 10.1128/jb.122.1.34-40.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Josefsson L. G., Randall L. L. Different exported proteins in E. coli show differences in the temporal mode of processing in vivo. Cell. 1981 Jul;25(1):151–157. doi: 10.1016/0092-8674(81)90239-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Löfdahl S., Guss B., Uhlén M., Philipson L., Lindberg M. Gene for staphylococcal protein A. Proc Natl Acad Sci U S A. 1983 Feb;80(3):697–701. doi: 10.1073/pnas.80.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney W. C., Hogg R. W., Hermodson M. A. The amino acid sequence of the D-galactose-binding protein from Escherichia coli B/r. J Biol Chem. 1981 May 10;256(9):4350–4356. [PubMed] [Google Scholar]
- Michel J. F., Millet J. Physiological studies on early-blocked sporulation mutants of Bacillus subtilis. J Appl Bacteriol. 1970 Mar;33(1):220–227. doi: 10.1111/j.1365-2672.1970.tb05246.x. [DOI] [PubMed] [Google Scholar]
- Norris K., Norris F., Christiansen L., Fiil N. Efficient site-directed mutagenesis by simultaneous use of two primers. Nucleic Acids Res. 1983 Aug 11;11(15):5103–5112. doi: 10.1093/nar/11.15.5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva I., Lehtovaara P., Käriäinen L., Sibakov M., Cantell K., Schein C. H., Kashiwagi K., Weissmann C. Secretion of interferon by Bacillus subtilis. Gene. 1983 May-Jun;22(2-3):229–235. doi: 10.1016/0378-1119(83)90107-5. [DOI] [PubMed] [Google Scholar]
- Palva I., Sarvas M., Lehtovaara P., Sibakov M., Käriäinen L. Secretion of Escherichia coli beta-lactamase from Bacillus subtilis by the aid of alpha-amylase signal sequence. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5582–5586. doi: 10.1073/pnas.79.18.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. A putative signal peptidase recognition site and sequence in eukaryotic and prokaryotic signal peptides. J Mol Biol. 1983 Jun 25;167(2):391–409. doi: 10.1016/s0022-2836(83)80341-6. [DOI] [PubMed] [Google Scholar]
- Saunders C. W., Schmidt B. J., Mirot M. S., Thompson L. D., Guyer M. S. Use of chromosomal integration in the establishment and expression of blaZ, a Staphylococcus aureus beta-lactamase gene, in Bacillus subtilis. J Bacteriol. 1984 Mar;157(3):718–726. doi: 10.1128/jb.157.3.718-726.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlén M., Guss B., Nilsson B., Gatenbeck S., Philipson L., Lindberg M. Complete sequence of the staphylococcal gene encoding protein A. A gene evolved through multiple duplications. J Biol Chem. 1984 Feb 10;259(3):1695–1702. [PubMed] [Google Scholar]
- Vasantha N., Freese E. Enzyme changes during Bacillus subtilis sporulation caused by deprivation of guanine nucleotides. J Bacteriol. 1980 Dec;144(3):1119–1125. doi: 10.1128/jb.144.3.1119-1125.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasantha N., Thompson L. D., Rhodes C., Banner C., Nagle J., Filpula D. Genes for alkaline protease and neutral protease from Bacillus amyloliquefaciens contain a large open reading frame between the regions coding for signal sequence and mature protein. J Bacteriol. 1984 Sep;159(3):811–819. doi: 10.1128/jb.159.3.811-819.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]