Abstract
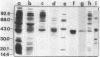
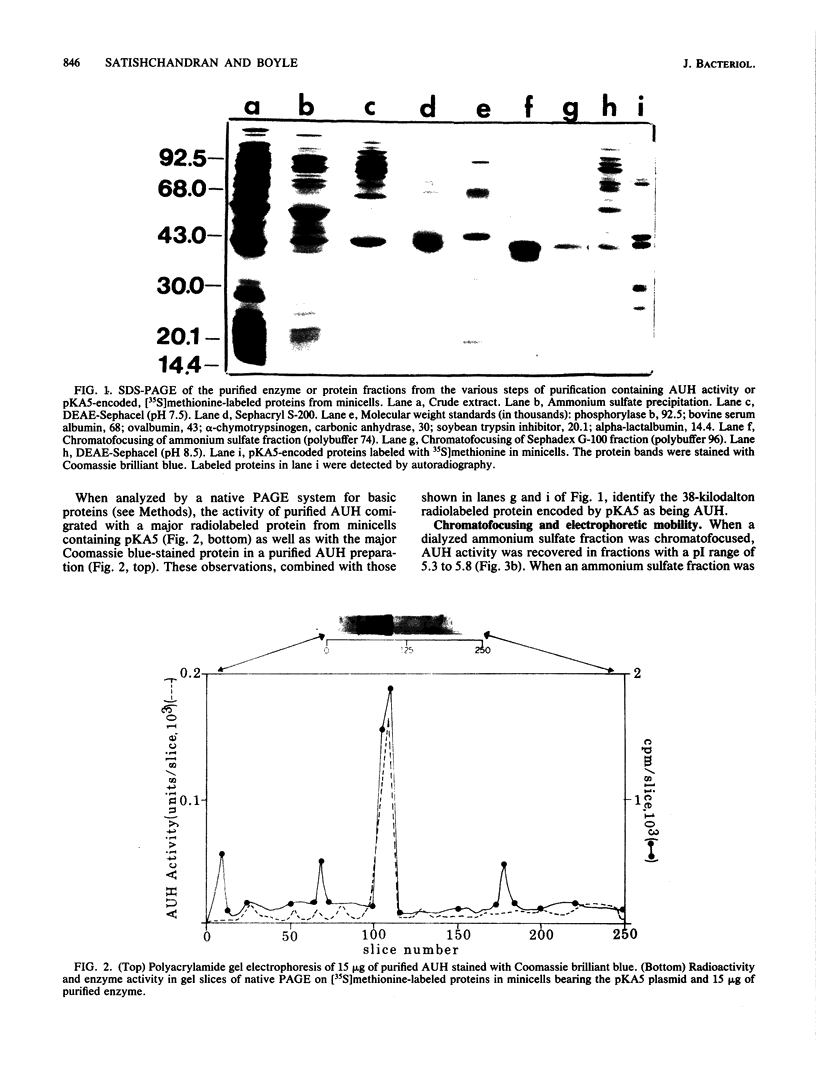
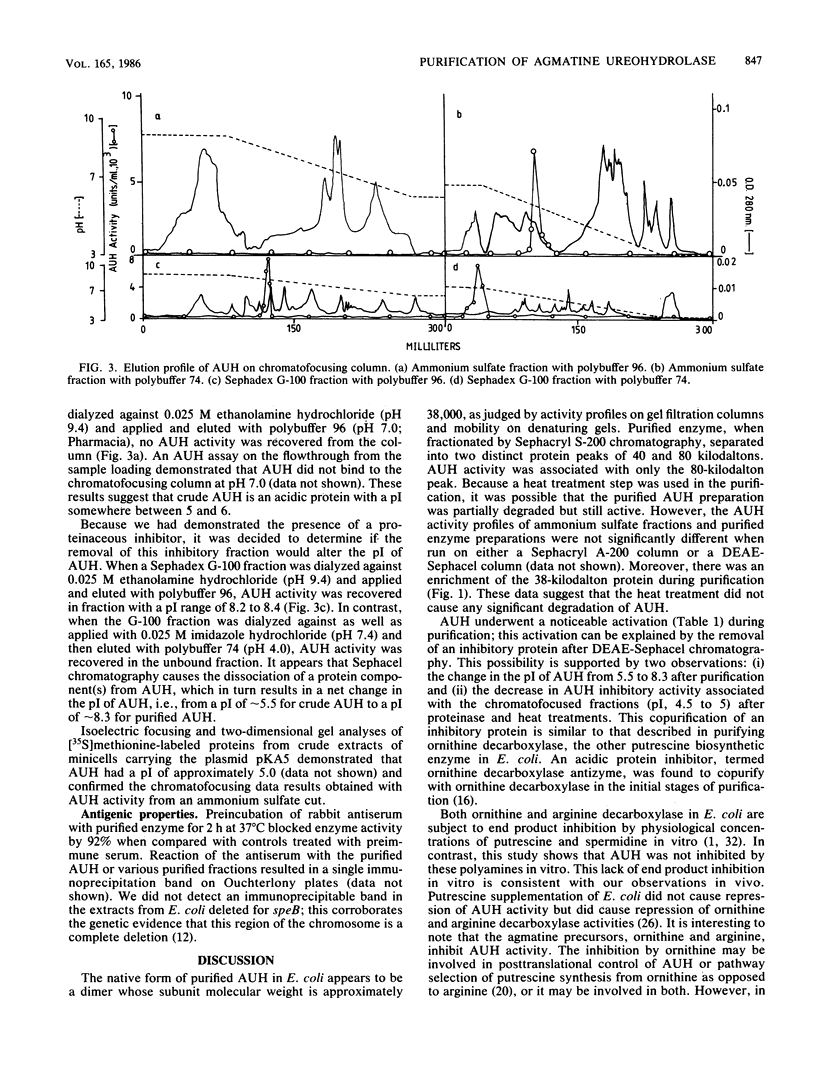
The putrescine biosynthetic enzyme agmatine ureohydrolase (AUH) (EC 3.5.3.11) catalyzes the conversion of agmatine to putrescine in Escherichia coli. AUH was purified approximately 1,600-fold from an E. coli strain transformed with the plasmid pKA5 bearing the speB gene encoding the enzyme. The purification procedure included ammonium sulfate precipitation, heat treatment, and DEAE-sephacel column chromatography. The molecular mass of nondenatured AUH is approximately 80,000 daltons as determined by gel-sieving column chromatography, while on denaturing polyacrylamide gels, the molecular mass is approximately 38,000 daltons; thus, native AUH is most likely a dimer. A radiolabeled protein extracted from minicells carrying the pKA5 plasmid comigrated with the purified AUH in both sodium dodecyl sulfate-polyacrylamide and native polyacrylamide gels. The pI of purified AUH is between 8.2 and 8.4, as determined by either chromatofocusing or isoelectric focusing. The Km of purified AUH for agmatine is 1.2 mM; the pH optimum is 7.3. Neither the numerous ions and nucleotides tested nor polyamines affected AUH activity in vitro. EDTA and EGTA [ethylene glycol-bis (beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid] at 1 mM inactivated AUH activity by 53 and 74%, respectively; none of numerous divalent cations tested restored AUH activity. Ornithine inhibited AUH activity noncompetitively (Ki = 6 X 10(-3) M), while arginine inhibited AUH activity competitively (Ki = 9 X 10(-3) M).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applebaum D. M., Dunlap J. C., Morris D. R. Comparison of the biosynthetic and biodegradative ornithine decarboxylases of Escherichia coli. Biochemistry. 1977 Apr 19;16(8):1580–1584. doi: 10.1021/bi00627a008. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Boyle S. M., Adachi K. Biosynthetic ornithine and arginine decarboxylases: correlation of rates of synthesis with activities in Escherichia coli during exponential growth and following nutritional shift-up. Can J Microbiol. 1982 Aug;28(8):945–950. doi: 10.1139/m82-142. [DOI] [PubMed] [Google Scholar]
- Boyle S. M., MacIntyre M. F., Sells B. H. Polyamine levels in Escherichia coli during nutritional shiftup and exponential growth. Biochim Biophys Acta. 1977 Aug 2;477(3):221–227. doi: 10.1016/0005-2787(77)90047-8. [DOI] [PubMed] [Google Scholar]
- Boyle S. M., Markham G. D., Hafner E. W., Wright J. M., Tabor H., Tabor C. W. Expression of the cloned genes encoding the putrescine biosynthetic enzymes and methionine adenosyltransferase of Escherichia coli (speA, speB, speC and metK). Gene. 1984 Oct;30(1-3):129–136. doi: 10.1016/0378-1119(84)90113-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Friedrich B., Magasanik B. Enzymes of agmatine degradation and the control of their synthesis in Klebsiella aerogenes. J Bacteriol. 1979 Mar;137(3):1127–1133. doi: 10.1128/jb.137.3.1127-1133.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich B., Magasanik B. Utilization of arginine by Klebsiella aerogenes. J Bacteriol. 1978 Feb;133(2):680–685. doi: 10.1128/jb.133.2.680-685.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner E. W., Tabor C. W., Tabor H. Isolation of a metK mutant with a temperature-sensitive S-adenosylmethionine synthetase. J Bacteriol. 1977 Dec;132(3):832–840. doi: 10.1128/jb.132.3.832-840.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller J. S., Rostomily R., Kyriakidis D. A., Canellakis E. S. Regulation of polyamine biosynthesis in Escherichia coli by basic proteins. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5181–5184. doi: 10.1073/pnas.80.17.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölttä E., Jänne J., Pispa J. Ornithine decarboxylase from Escherichia coli: stimulation of the enzyme activity by nucleotides. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1165–1171. doi: 10.1016/0006-291x(72)90957-6. [DOI] [PubMed] [Google Scholar]
- Khramov V. A. Vliianie spirtov na aktivnost' bakterial'noi agmatinazy. Biokhimiia. 1978;43(4):609–613. [PubMed] [Google Scholar]
- Kyriakidis D. A., Heller J. S., Canellakis E. S. Modulation of ornithine decarboxylase activity in Escherichia coli by positive and negative effectors. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4699–4703. doi: 10.1073/pnas.75.10.4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morris D. R., Fillingame R. H. Regulation of amino acid decarboxylation. Annu Rev Biochem. 1974;43(0):303–325. doi: 10.1146/annurev.bi.43.070174.001511. [DOI] [PubMed] [Google Scholar]
- Morris D. R., Jorstad C. M. Isolation of conditionally putrescine-deficient mutants of Escherichia coli. J Bacteriol. 1970 Mar;101(3):731–737. doi: 10.1128/jb.101.3.731-737.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. R., Koffron K. L. Urea production and putrescine biosynthesis by Escherichia coli. J Bacteriol. 1967 Nov;94(5):1516–1519. doi: 10.1128/jb.94.5.1516-1519.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T. T., Cohen S. S. Regulation of ornithine decarboxylase activity by guanine nucleotides: in vivo test in potassium-depleted Escherichia coli. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3502–3505. doi: 10.1073/pnas.73.10.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satishchandran C., Boyle S. M. Antagonistic transcriptional regulation of the putrescine biosynthetic enzyme agmatine ureohydrolase by cyclic AMP and agmatine in Escherichia coli. J Bacteriol. 1984 Feb;157(2):552–559. doi: 10.1128/jb.157.2.552-559.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer R. C., 3rd, Merril C. R., Shifrin S. A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels. Anal Biochem. 1979 Sep 15;98(1):231–237. doi: 10.1016/0003-2697(79)90732-2. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H., Hafner E. W., Markham G. D., Boyle S. M. Cloning of the Escherichia coli genes for the biosynthetic enzymes for polyamines. Methods Enzymol. 1983;94:117–121. doi: 10.1016/s0076-6879(83)94019-3. [DOI] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Formation of 1,4-diaminobutane and of spermidine by an ornithine auxotroph of Escherichia coli grown on limiting ornithine or arginine. J Biol Chem. 1969 May 10;244(9):2286–2292. [PubMed] [Google Scholar]
- Wright J. M., Boyle S. M. Negative control of ornithine decarboxylase and arginine decarboxylase by adenosine-3':5'-cyclic monophosphate in Escherichia coli. Mol Gen Genet. 1982;186(4):482–487. doi: 10.1007/BF00337952. [DOI] [PubMed] [Google Scholar]
- Wu W. H., Morris D. R. Biosynthetic arginine decarboxylase from Escherichia coli. Purification and properties. J Biol Chem. 1973 Mar 10;248(5):1687–1695. [PubMed] [Google Scholar]
- Wu W. H., Morris D. R. Biosynthetic arginine decarboxylase from Escherichia coli. Subunit interactions and the role of magnesium ion. J Biol Chem. 1973 Mar 10;248(5):1696–1699. [PubMed] [Google Scholar]