Abstract
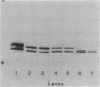
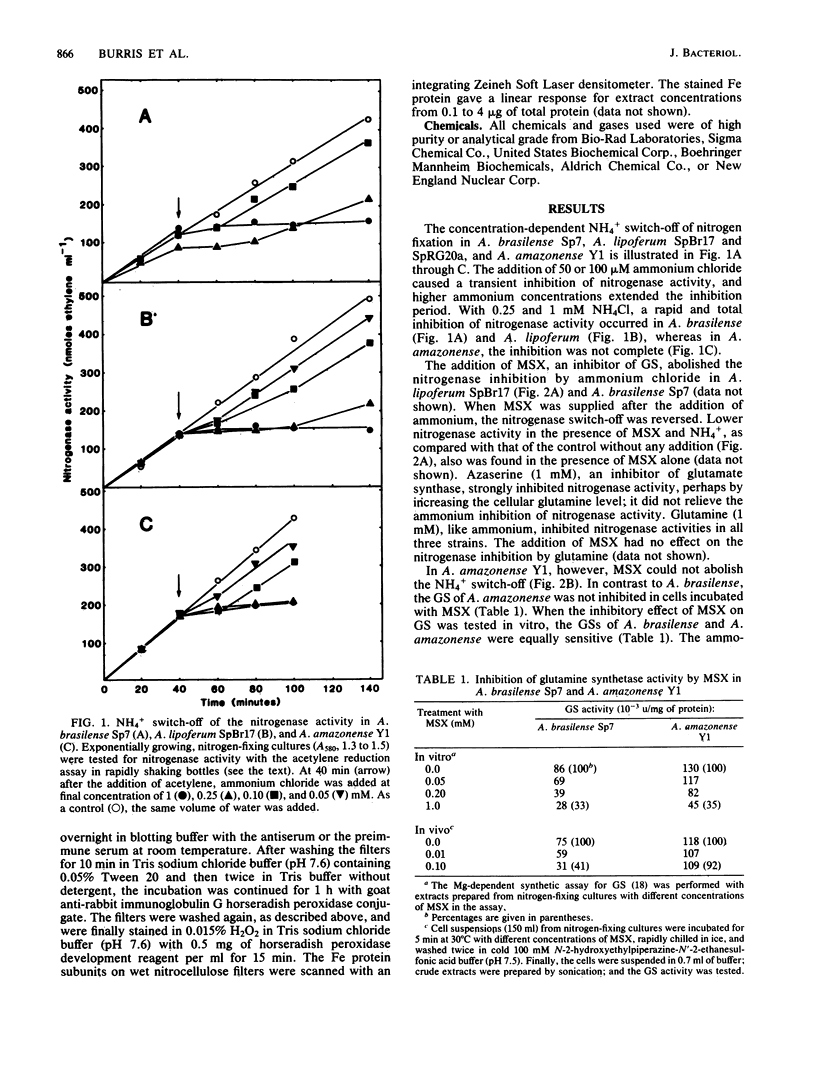
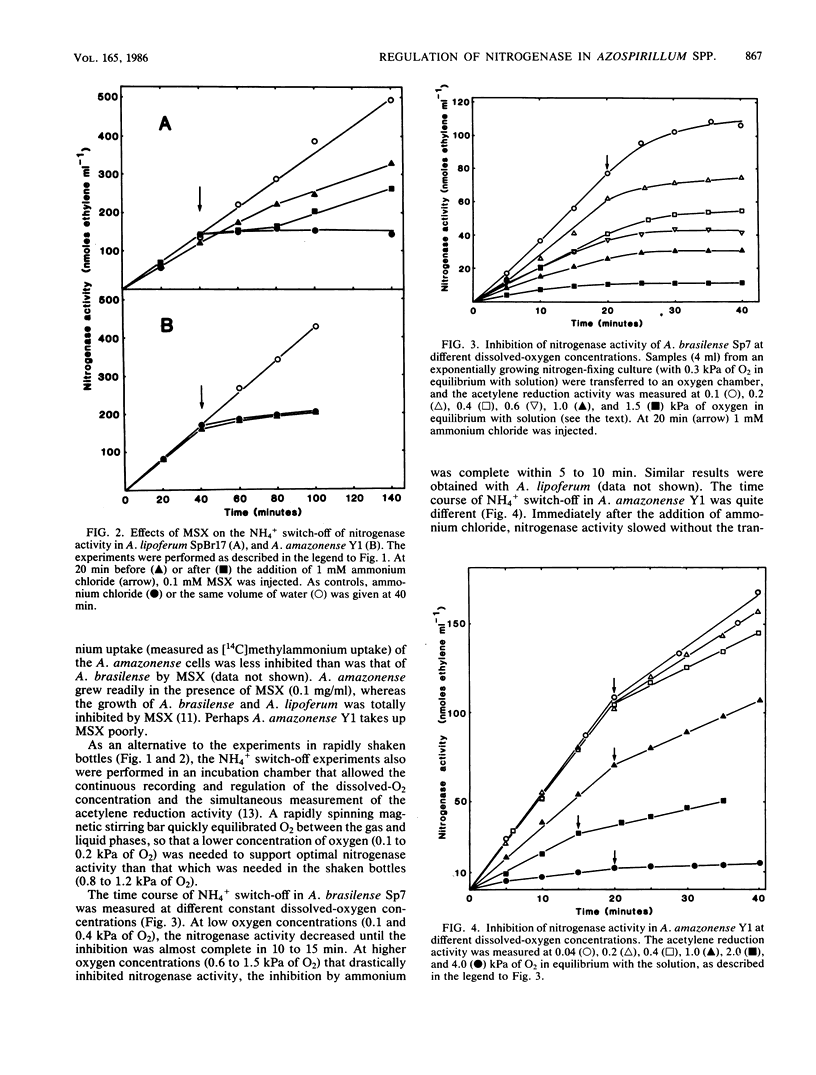
Ammonium chloride (greater than or equal to 0.05 mM) effectively and reversibly inhibited the nitrogenase activity of Azospirillum brasilense, Azospirillum lipoferum and Azospirillum amazonense. The glutamine synthetase inhibitor L-methionine-DL- sulfoximine abolished this "switch-off" in A. lipoferum and A. brasilense, but not in A. amazonense. Azaserine, an inhibitor of glutamate synthase, inhibited nitrogenase activity itself. This provides further evidence for glutamine as a metabolite of regulatory importance in the NH4+ switch-off phenomenon. In A. brasilense and A. lipoferum, a transition period before the complete inhibition of nitrogenase activity after the addition of 1 mM ammonium chloride was observed. The in vitro nitrogenase activity also was decreased after treatment with ammonium. During sodium dodecyl sulfate-polyacrylamide gel electrophoresis, a second dinitrogenase reductase (Fe protein) subunit appeared, which migrated in coincidence with the modified subunit of the inactive Fe protein of the nitrogenase of Rhodospirillum rubrum. After the addition of ammonium 32P was incorporated into this subunit of the Fe protein of A. brasilense. In A. amazonense, the inhibition of nitrogenase activity by ammonium was only partial, and no transition period could be observed. The in vitro nitrogenase activity of ammonium-treated cells was not decreased, and no evidence for a modified Fe protein subunit was found. Nitrogenase extracts of A. amazonense were active and had an Fe protein that migrated as a close double band on sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burris R. H. Nitrogen fixation--assay methods and techniques. Methods Enzymol. 1972;24:415–431. doi: 10.1016/0076-6879(72)24088-5. [DOI] [PubMed] [Google Scholar]
- Cejudo F. J., de la Torre A., Paneque A. Short-term ammonium inhibition of nitrogen fixation in Azotobacter. Biochem Biophys Res Commun. 1984 Sep 17;123(2):431–437. doi: 10.1016/0006-291x(84)90248-1. [DOI] [PubMed] [Google Scholar]
- Cullen S. E., Schwartz B. D. An improved method for isolation of H-2 and Ia alloantigens with immunoprecipitation induced by protein A-bearing staphylococci. J Immunol. 1976 Jul;117(1):136–142. [PubMed] [Google Scholar]
- GOA J. A micro biuret method for protein determination; determination of total protein in cerebrospinal fluid. Scand J Clin Lab Invest. 1953;5(3):218–222. doi: 10.3109/00365515309094189. [DOI] [PubMed] [Google Scholar]
- Gordon J. K., Shah V. K., Brill W. J. Feedback inhibition of nitrogenase. J Bacteriol. 1981 Dec;148(3):884–888. doi: 10.1128/jb.148.3.884-888.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotto J. W., Yoch D. C. Regulation of nitrogenase activity by covalent modification in Chromatium vinosum. Arch Microbiol. 1985 Feb;141(1):40–43. doi: 10.1007/BF00446737. [DOI] [PubMed] [Google Scholar]
- Hochman A., Burris R. H. Effect of oxygen on acetylene reduction by photosynthetic bacteria. J Bacteriol. 1981 Aug;147(2):492–499. doi: 10.1128/jb.147.2.492-499.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemoto R. H., Ludden P. W. Effect of ammonia, darkness, and phenazine methosulfate on whole-cell nitrogenase activity and Fe protein modification in Rhodospirillum rubrum. J Bacteriol. 1984 May;158(2):713–720. doi: 10.1128/jb.158.2.713-720.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner D., Alef K., Hartmann A. Uptake of methionine sulfoximine by some N2 fixing bacteria, and its effect on ammonium transport. FEBS Lett. 1983 Nov 28;164(1):121–123. doi: 10.1016/0014-5793(83)80032-5. [DOI] [PubMed] [Google Scholar]
- Klugkist J., Haaker H. Inhibition of nitrogenase activity by ammonium chloride in Azotobacter vinelandii. J Bacteriol. 1984 Jan;157(1):148–151. doi: 10.1128/jb.157.1.148-151.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugkist J., Haaker H., Wassink H., Veeger C. The catalytic activity of nitrogenase in intact Azotobacter vinelandii cells. Eur J Biochem. 1985 Feb 1;146(3):509–515. doi: 10.1111/j.1432-1033.1985.tb08681.x. [DOI] [PubMed] [Google Scholar]
- Laane C., Krone W., Konings W., Haaker H., Veeger C. Short-term effect of ammonium chloride on nitrogen fixation by Azotobacter vinelandii and by bacteroids of Rhizobium leguminosarum. Eur J Biochem. 1980 Jan;103(1):39–46. doi: 10.1111/j.1432-1033.1980.tb04286.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ludden P. W., Okon Y., Burris R. H. The nitrogenase system of Spirillum lipoferum. Biochem J. 1978 Sep 1;173(3):1001–1003. doi: 10.1042/bj1731001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyra C. A., Van Berkum P. Nitrate reduction nitrogenase activity in Spirillum lipoferum1. Can J Microbiol. 1977 Mar;23(3):306–310. doi: 10.1139/m77-045. [DOI] [PubMed] [Google Scholar]
- Okon Y., Albrecht S. L., Burris R. H. Carbon and ammonia metabolism of Spirillum lipoferum. J Bacteriol. 1976 Nov;128(2):592–597. doi: 10.1128/jb.128.2.592-597.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okon Y., Houchins J. P., Albrecht S. L., Burris R. H. Growth of Spirillum lipoferum at constant partial pressures of oxygen, and the properties of its nitrogenase in cell-free extracts. J Gen Microbiol. 1977 Jan;98(1):87–93. doi: 10.1099/00221287-98-1-87. [DOI] [PubMed] [Google Scholar]
- Pope M. R., Murrell S. A., Ludden P. W. Covalent modification of the iron protein of nitrogenase from Rhodospirillum rubrum by adenosine diphosphoribosylation of a specific arginine residue. Proc Natl Acad Sci U S A. 1985 May;82(10):3173–3177. doi: 10.1073/pnas.82.10.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saari L. L., Triplett E. W., Ludden P. W. Purification and properties of the activating enzyme for iron protein of nitrogenase from the photosynthetic bacterium Rhodospirillum rubrum. J Biol Chem. 1984 Dec 25;259(24):15502–15508. [PubMed] [Google Scholar]
- Song S. D., Hartmann A., Burris R. H. Purification and properties of the nitrogenase of Azospirillum amazonense. J Bacteriol. 1985 Dec;164(3):1271–1277. doi: 10.1128/jb.164.3.1271-1277.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet W. J., Burris R. H. Inhibition of nitrogenase activity by NH+4 in Rhodospirillum rubrum. J Bacteriol. 1981 Feb;145(2):824–831. doi: 10.1128/jb.145.2.824-831.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrand J. J., Krieg N. R., Döbereiner J. A taxonomic study of the Spirillum lipoferum group, with descriptions of a new genus, Azospirillum gen. nov. and two species, Azospirillum lipoferum (Beijerinck) comb. nov. and Azospirillum brasilense sp. nov. Can J Microbiol. 1978 Aug;24(8):967–980. doi: 10.1139/m78-160. [DOI] [PubMed] [Google Scholar]
- Zumft W. G., Castillo F. Regulatory properties of the nitrogenase from Rhodopseudomonas palustris. Arch Microbiol. 1978 Apr 27;117(1):53–60. doi: 10.1007/BF00689351. [DOI] [PubMed] [Google Scholar]