Abstract
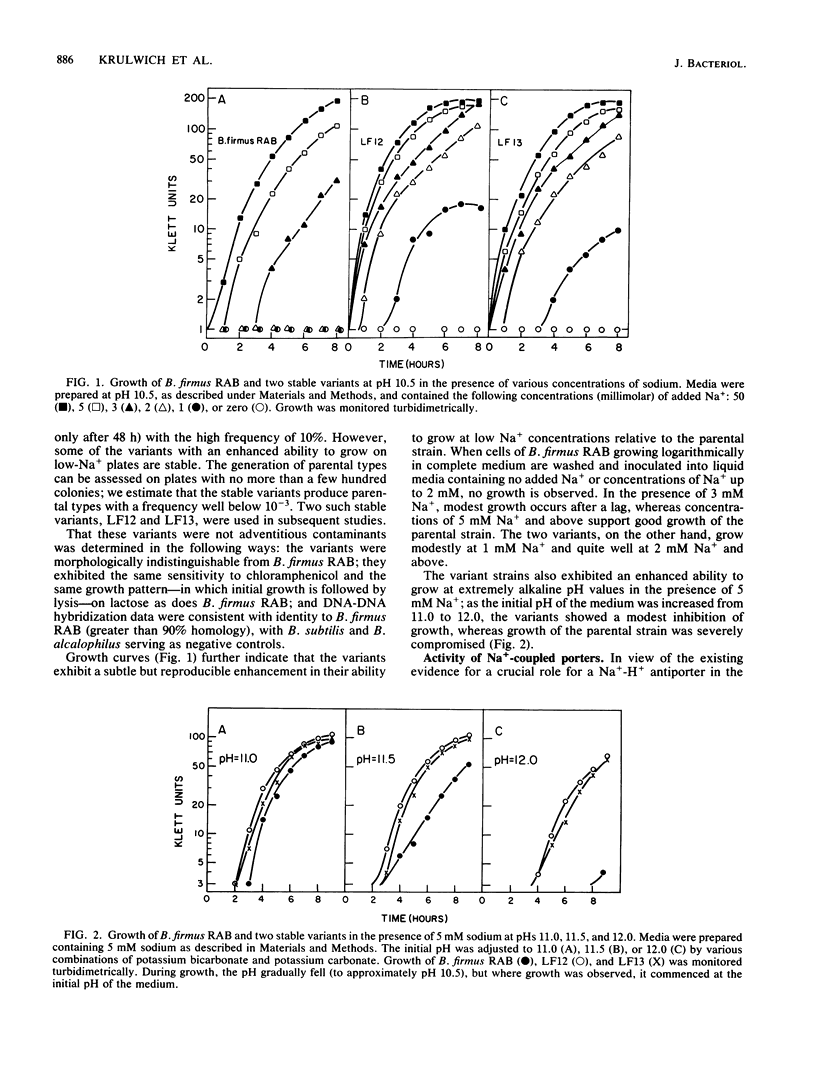
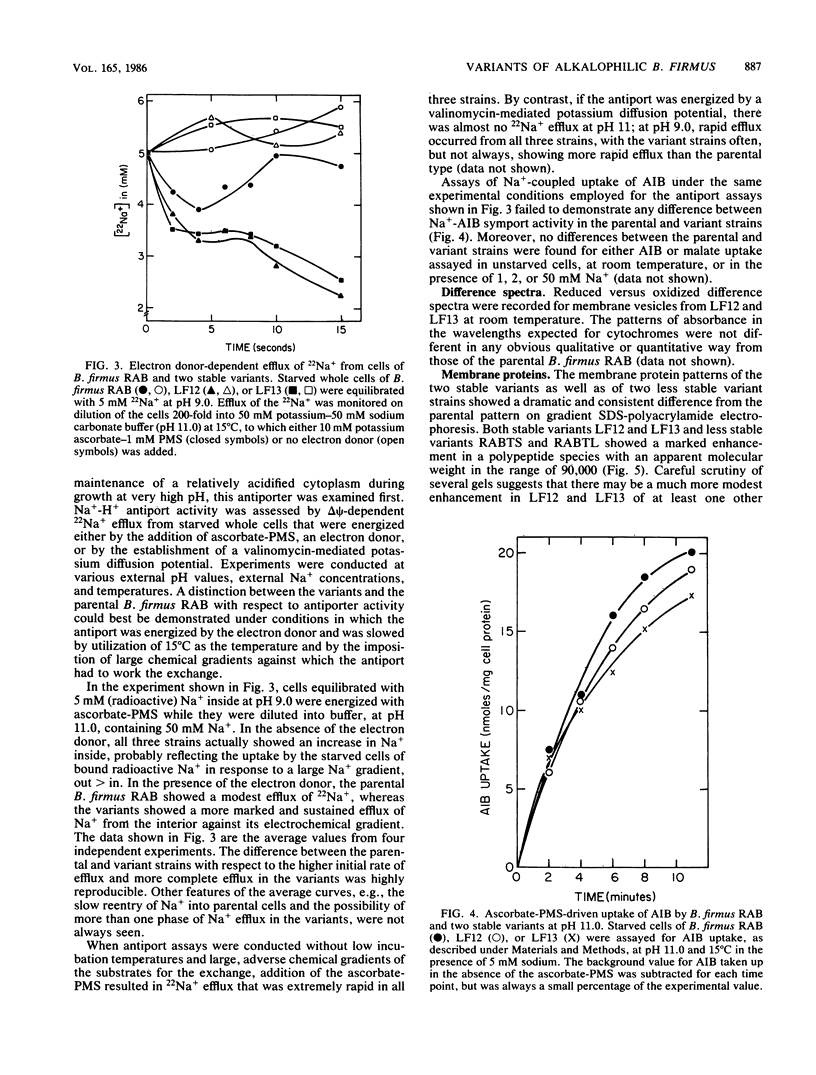
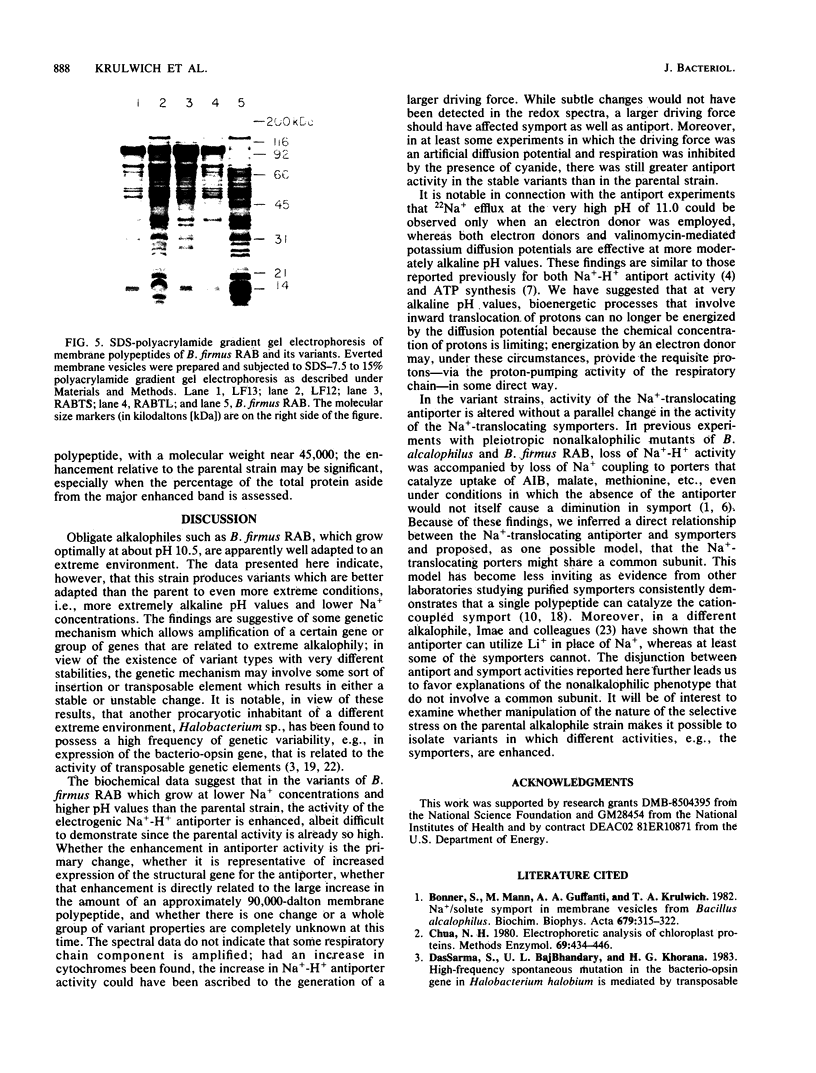
Obligately alkalophilic Bacillus firmus RAB cannot grow well on media containing less than 5 mM Na+. However, variant strains can be isolated on plates containing 2 to 3 mM Na+. These variants are observed only rarely in cultures that are plated before being subjected to repeated transfers in liquid medium. Cultures which have been transferred several times produce variants at an apparent frequency of 2 X 10(-4). Most of these variants are unstable, generating parental types at the high frequency of 10%; however, stable variants can be isolated. These strains grow better than the parental strain at very high pH values in the presence of 5 mM Na+ and have enhanced activity of the Na+ -H+ antiporter that has been implicated in pH homeostasis. By contrast, Na+ -coupled solute uptake is indistinguishable from that of the parental strain, and no obvious changes in the respiratory chain components are apparent in reduced versus oxidized difference spectra. The membranes of the variants show a marked enhancement, on sodium dodecyl sulfate-polyacrylamide gradient electrophoresis, in one polypeptide band with a molecular weight in the range of 90,000. The findings are discussed from the point of view of genetic mechanisms that might confer adaptability to even more extreme environments than usual and in view of earlier models relating the Na+ -translocating activities of the alkalophiles.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DasSarma S., RajBhandary U. L., Khorana H. G. High-frequency spontaneous mutation in the bacterio-opsin gene in Halobacterium halobium is mediated by transposable elements. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2201–2205. doi: 10.1073/pnas.80.8.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M. L., Guffanti A. A., Krulwich T. A. Characterization of the Na+/H+ antiporter of alkalophilic bacilli in vivo: delta psi-dependent 22Na+ efflux from whole cells. J Bacteriol. 1983 Dec;156(3):1151–1157. doi: 10.1128/jb.156.3.1151-1157.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guffanti A. A., Cohn D. E., Kaback H. R., Krulwich T. A. Relationship between the Na+/H+ antiporter and Na+/substrate symport in Bacillus alcalophilus. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1481–1484. doi: 10.1073/pnas.78.3.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guffanti A. A., Fuchs R. T., Schneier M., Chiu E., Krulwich T. A. A transmembrane electrical potential generated by respiration is not equivalent to a diffusion potential of the same magnitude for ATP synthesis by Bacillus firmus RAB. J Biol Chem. 1984 Mar 10;259(5):2971–2975. [PubMed] [Google Scholar]
- Guffanti A. A., Susman P., Blanco R., Krulwich T. A. The protonmotive force and alpha-aminoisobutyric acid transport in an obligately alkalophilic bacterium. J Biol Chem. 1978 Feb 10;253(3):708–715. [PubMed] [Google Scholar]
- Hegeman G. D. Synthesis of the enzymes of the mandelate pathway by Pseudomonas putida. I. Synthesis of enzymes by the wild type. J Bacteriol. 1966 Mar;91(3):1140–1154. doi: 10.1128/jb.91.3.1140-1154.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H., Kambe T., Kagawa Y. A purified alanine carrier composed of a single polypeptide from thermophilic bacterium PS3 driven by either proton or sodium ion gradient. J Biol Chem. 1984 Sep 10;259(17):10653–10656. [PubMed] [Google Scholar]
- Krulwich T. A., Guffanti A. A., Bornstein R. F., Hoffstein J. A sodium requirement for growth, solute transport, and pH homeostasis in Bacillus firmus RAB. J Biol Chem. 1982 Feb 25;257(4):1885–1889. [PubMed] [Google Scholar]
- Krulwich T. A., Guffanti A. A. Physiology of acidophilic and alkalophilic bacteria. Adv Microb Physiol. 1983;24:173–214. doi: 10.1016/s0065-2911(08)60386-0. [DOI] [PubMed] [Google Scholar]
- Kudo T., Kato C., Horikoshi K. Excretion of the penicillinase of an alkalophilic Bacillus sp. through the Escherichia coli outer membrane. J Bacteriol. 1983 Nov;156(2):949–951. doi: 10.1128/jb.156.2.949-951.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. J., Belkina S., Krulwich T. A. Alkalophiles have much higher cytochrome contents than conventional bacteria and than their own non-alkalophilic mutant derivatives. Biochem Biophys Res Commun. 1980 Jul 31;95(2):857–863. doi: 10.1016/0006-291x(80)90866-9. [DOI] [PubMed] [Google Scholar]
- Newman M. J., Foster D. L., Wilson T. H., Kaback H. R. Purification and reconstitution of functional lactose carrier from Escherichia coli. J Biol Chem. 1981 Nov 25;256(22):11804–11808. [PubMed] [Google Scholar]
- Pfeifer F., Weidinger G., Goebel W. Genetic variability in Halobacterium halobium. J Bacteriol. 1981 Jan;145(1):375–381. doi: 10.1128/jb.145.1.375-381.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sashihara N., Kudo T., Horikoshi K. Molecular cloning and expression of cellulase genes of alkalophilic Bacillus sp. strain N-4 in Escherichia coli. J Bacteriol. 1984 May;158(2):503–506. doi: 10.1128/jb.158.2.503-506.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher B. M., Dean D. H., Garro A. J. Fragmentation of Bacillus bacteriophage phi105 DNA by complementary single-stranded DNA in the cohesive ends of the molecule. J Virol. 1977 Aug;23(2):377–383. doi: 10.1128/jvi.23.2.377-383.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsek M., DasSarma S., RajBhandary U. L., Khorana H. G. A transposable element from Halobacterium halobium which inactivates the bacteriorhodopsin gene. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7268–7272. doi: 10.1073/pnas.79.23.7268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama S., Matsukura H., Imae Y. Relationship between Na+-dependent cytoplasmic pH homeostasis and Na+-dependent flagellar rotation and amino acid transport in alkalophilic Bacillus. FEBS Lett. 1985 Mar 25;182(2):265–268. doi: 10.1016/0014-5793(85)80312-4. [DOI] [PubMed] [Google Scholar]
- Takinishi H., Sekiguchi T., Koyama N., Shishido K., Nosoh Y. DNA from alkalophilic Bacillus can transform B. subtilis to alkalophily. FEBS Lett. 1983 Apr 5;154(1):201–204. doi: 10.1016/0014-5793(83)80903-x. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]