Abstract
Irradiation of the photoheterotrophic cyanobacterium Synechocystis sp. PCC 6803 with low levels of UV light allows for stable, integrative transformation of these cells by heterologous DNA. In this system, transformation does not rely on an autonomously replicating plasmid and is independent of homologous recombination. Cells treated with UV light in the absence of DNA and cells given DNA but not exposed to UV do not yield antibiotic-resistant colonies in platings of up to 2 X 10(8) cells. Optimal conditions for this UV-induced transformation are described. Analysis of the transformants indicates that (i) only a segment of the introduced plasmid is found in the DNA of the transformed cells; (ii) in independently isolated clones, DNA insertion apparently occurs at different sites in the chromosome; and (iii) hybridization data suggest that insertion in one of the transformants may have occurred into a region of the chromosome that is repeated or that integration of plasmid DNA may have been accompanied by a rearrangement or duplication of DNA sequences near the insertion site. DNA isolated from the primary transformants as well as a cloned fragment containing the UV-inserted plasmid sequence and flanking cyanobacterial DNA transform wild-type cells at a high frequency (5.0 X 10(-4) and 1.5 X 10(-5), respectively). Possible mechanisms of this transformation system are discussed, as are the potential uses of this system as an integrative cloning-complementation vector and as a mutagenic agent in which the genetic lesion is already tagged with a selectable marker.
Full text
PDF


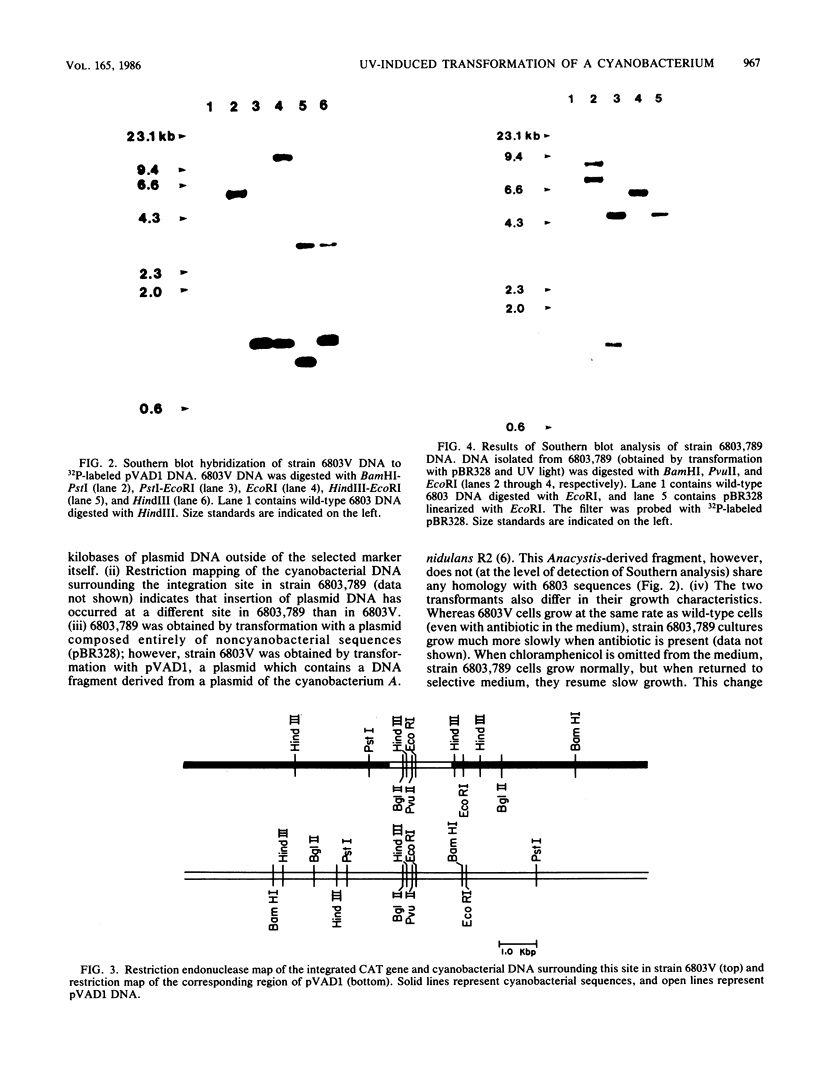

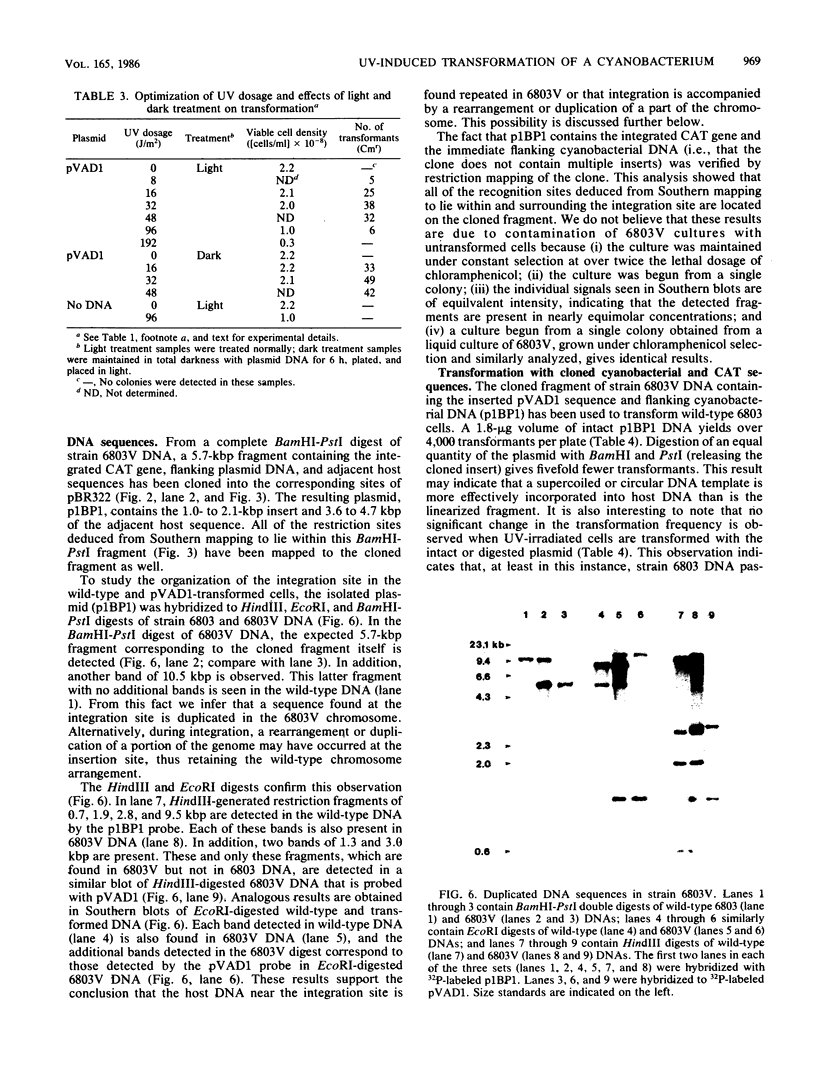
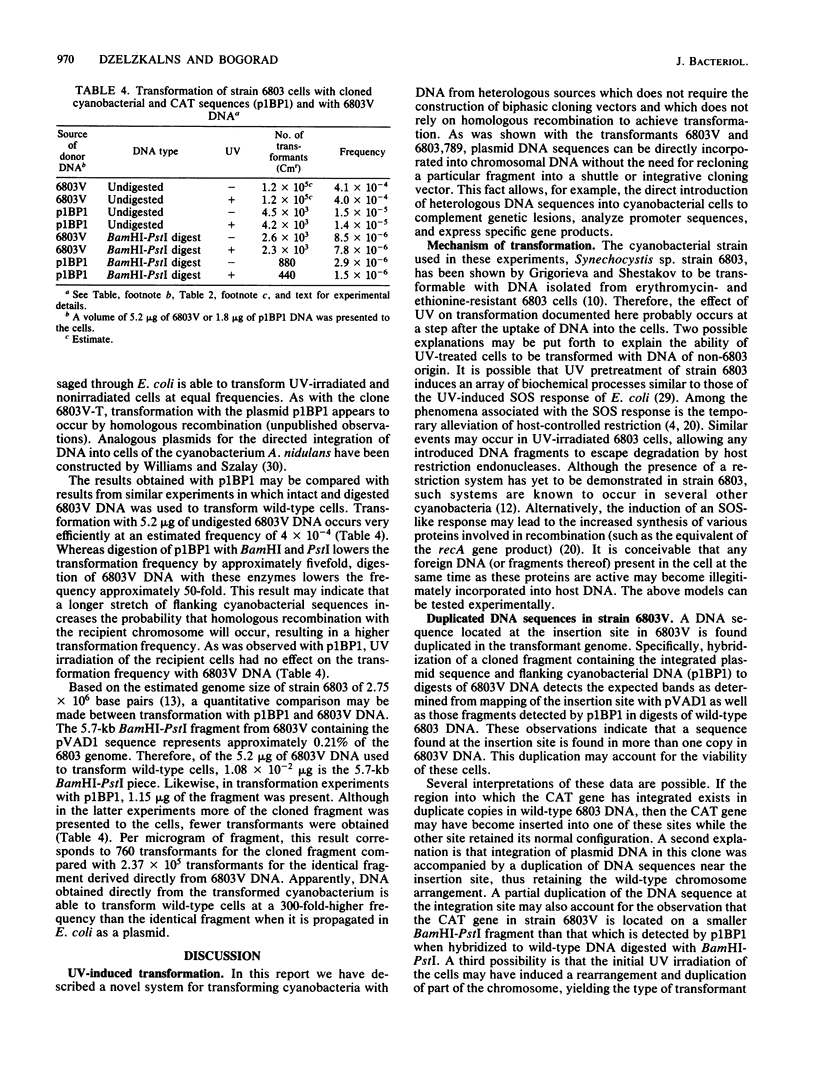

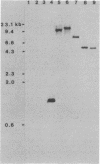
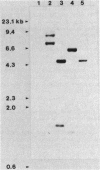
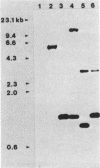
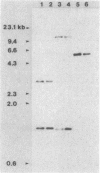
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. P., Roth J. R. Tandem genetic duplications in phage and bacteria. Annu Rev Microbiol. 1977;31:473–505. doi: 10.1146/annurev.mi.31.100177.002353. [DOI] [PubMed] [Google Scholar]
- Astier C., Elmorjani K., Meyer I., Joset F., Herdman M. Photosynthetic mutants of the cyanobacteria Synechocystis sp. strains PCC 6714 and PCC 6803: sodium p-hydroxymercuribenzoate as a selective agent. J Bacteriol. 1984 May;158(2):659–664. doi: 10.1128/jb.158.2.659-664.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzby J. S., Porter R. D., Stevens S. E., Jr Plasmid transformation in Agmenellum quadruplicatum PR-6: construction of biphasic plasmids and characterization of their transformation properties. J Bacteriol. 1983 Jun;154(3):1446–1450. doi: 10.1128/jb.154.3.1446-1450.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day R. S., 3rd UV-induced alleviation of K-specific restriction of bacteriophage lambda. J Virol. 1977 Mar;21(3):1249–1251. doi: 10.1128/jvi.21.3.1249-1251.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilly C. I., Houghton J. A. A study of genetic transformation in Gloeocapsa alpicola. J Gen Microbiol. 1977 Jan;98(1):277–280. doi: 10.1099/00221287-98-1-277. [DOI] [PubMed] [Google Scholar]
- Dzelzkalns V. A., Owens G. C., Bogorad L. Chloroplast promoter driven expression of the chloramphenicol acetyl transferase gene in a cyanobacterium. Nucleic Acids Res. 1984 Dec 11;12(23):8917–8925. doi: 10.1093/nar/12.23.8917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg D., Seijffers J. A new hybrid plasmid capable of transforming Escherichia coli and Anacystis nidulans. Gene. 1983 May-Jun;22(2-3):267–275. doi: 10.1016/0378-1119(83)90111-7. [DOI] [PubMed] [Google Scholar]
- Gendel S., Straus N., Pulleyblank D., Williams J. Shuttle cloning vectors for the cyanobacterium Anacystis nidulans. J Bacteriol. 1983 Oct;156(1):148–154. doi: 10.1128/jb.156.1.148-154.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Sherman L. A. A hybrid plasmid is a stable cloning vector for the cyanobacterium Anacystis nidulans R2. J Bacteriol. 1983 Sep;155(3):966–972. doi: 10.1128/jb.155.3.966-972.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. D., Mount D. W. Mechanisms of DNA replication and mutagenesis in ultraviolet-irradiated bacteria and mammalian cells. Prog Nucleic Acid Res Mol Biol. 1981;25:53–126. doi: 10.1016/s0079-6603(08)60483-3. [DOI] [PubMed] [Google Scholar]
- Herrera-Estrella L., Van den Broeck G., Maenhaut R., Van Montagu M., Schell J., Timko M., Cashmore A. Light-inducible and chloroplast-associated expression of a chimaeric gene introduced into Nicotiana tabacum using a Ti plasmid vector. Nature. 1984 Jul 12;310(5973):115–120. doi: 10.1038/310115a0. [DOI] [PubMed] [Google Scholar]
- Howley P. M., Israel M. A., Law M. F., Martin M. A. A rapid method for detecting and mapping homology between heterologous DNAs. Evaluation of polyomavirus genomes. J Biol Chem. 1979 Jun 10;254(11):4876–4883. [PubMed] [Google Scholar]
- Kuhlemeier C. J., Borrias W. E., van den Hondel C. A., van Arkel G. A. Vectors for cloning in cyanobacteria: construction and characterization of two recombinant plasmids capable of transformation of Escherichia coli K12 and Anacystis nidulans R2. Mol Gen Genet. 1981;184(2):249–254. doi: 10.1007/BF00272912. [DOI] [PubMed] [Google Scholar]
- Langridge J., Langridge P., Bergquist P. L. Extraction of nucleic acids from agarose gels. Anal Biochem. 1980 Apr;103(2):264–271. doi: 10.1016/0003-2697(80)90266-3. [DOI] [PubMed] [Google Scholar]
- Legerski R. J., Robberson D. L. Analysis and optimization of recombinant DNA joining reactions. J Mol Biol. 1985 Jan 20;181(2):297–312. doi: 10.1016/0022-2836(85)90093-2. [DOI] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Mulligan B., Schultes N., Chen L., Bogorad L. Nucleotide sequence of a multiple-copy gene for the B protein of photosystem II of a cyanobacterium. Proc Natl Acad Sci U S A. 1984 May;81(9):2693–2697. doi: 10.1073/pnas.81.9.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkwiszewski K. G., Kaney A. R. Genetic transformation of the blue-green bacterium, Anacystis nidulans. Arch Mikrobiol. 1974 Jun 7;98(1):31–37. doi: 10.1007/BF00425265. [DOI] [PubMed] [Google Scholar]
- Shestakov S. V., Khyen N. T. Evidence for genetic transformation in blue-green alga Anacystis nidulans. Mol Gen Genet. 1970;107(4):372–375. doi: 10.1007/BF00441199. [DOI] [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stevens S. E., Porter R. D. Transformation in Agmenellum quadruplicatum. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6052–6056. doi: 10.1073/pnas.77.10.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. G., Szalay A. A. Stable integration of foreign DNA into the chromosome of the cyanobacterium Synechococcus R2. Gene. 1983 Sep;24(1):37–51. doi: 10.1016/0378-1119(83)90129-4. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk C. P., Vonshak A., Kehoe P., Elhai J. Construction of shuttle vectors capable of conjugative transfer from Escherichia coli to nitrogen-fixing filamentous cyanobacteria. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1561–1565. doi: 10.1073/pnas.81.5.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]