Abstract
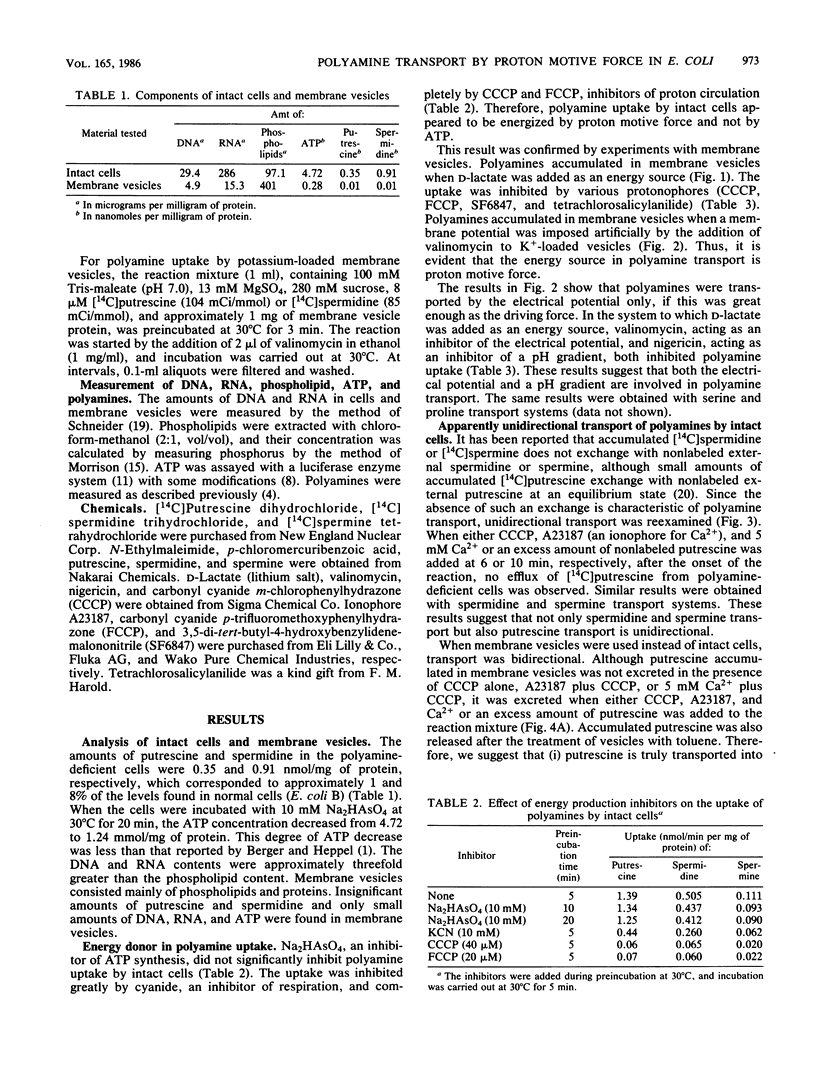
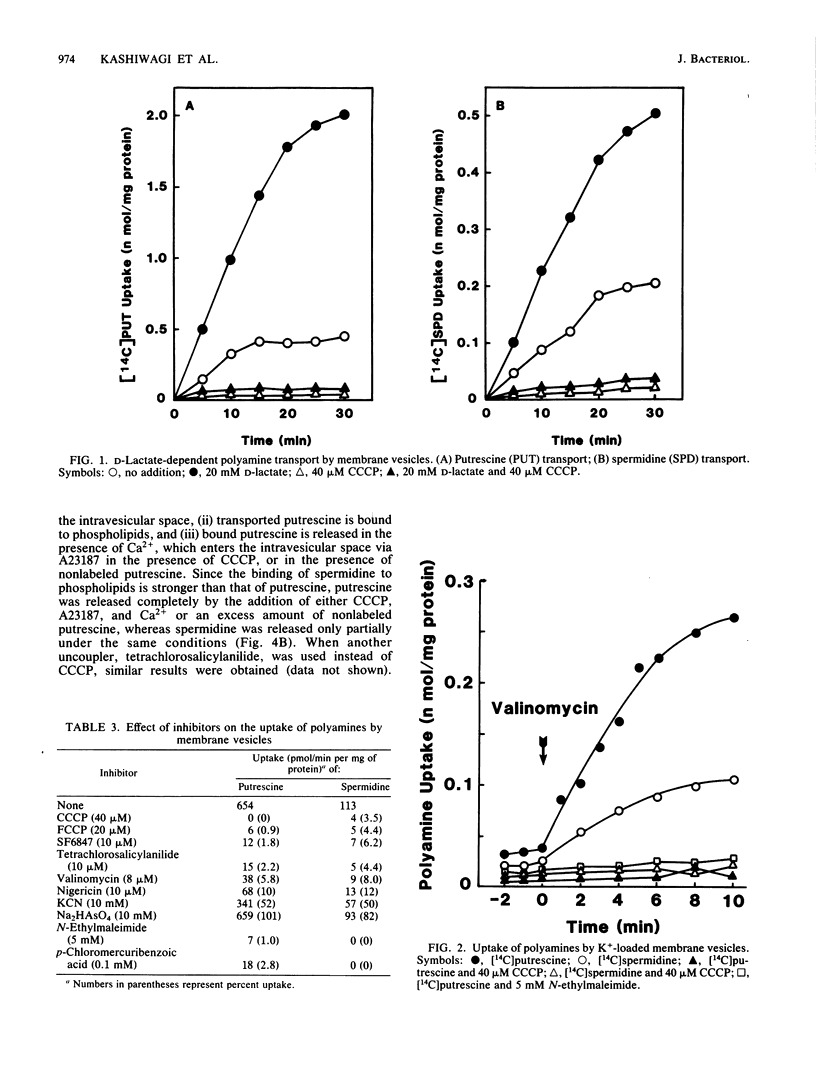
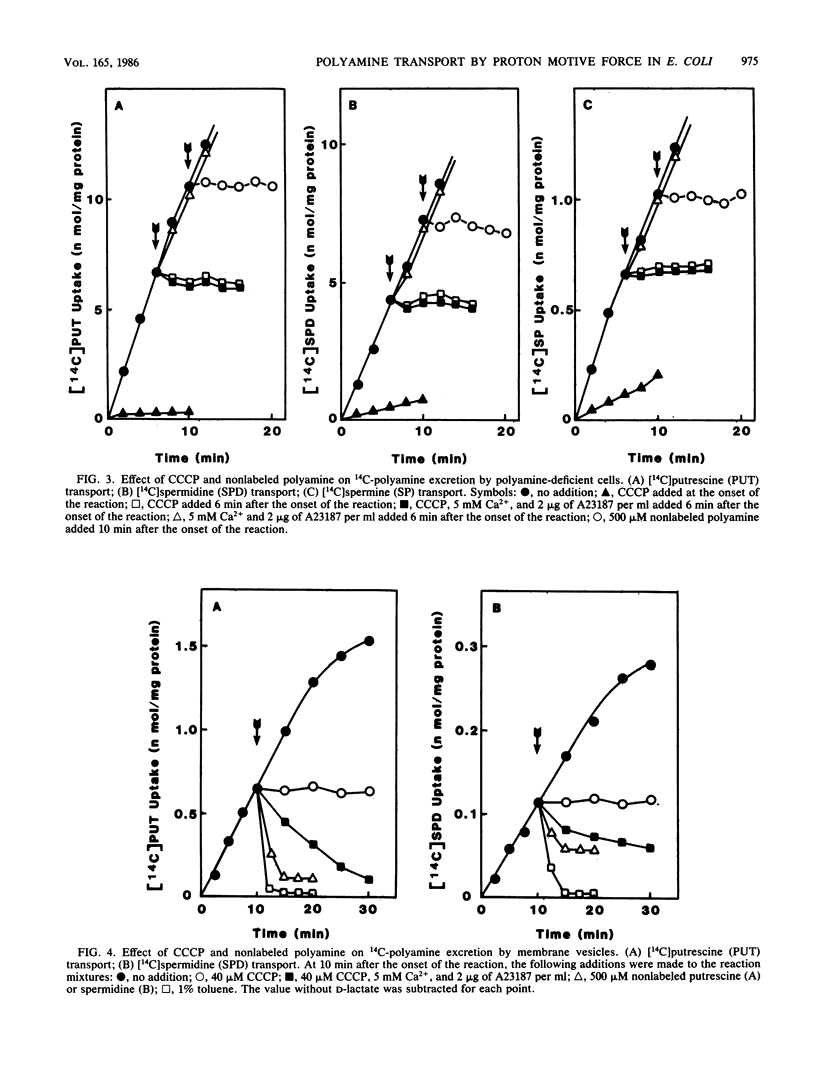
A transport system for polyamines was studied with both intact cells and membrane vesicles of an Escherichia coli polyamine-deficient mutant. Polyamine uptake by intact cells and membrane vesicles was inhibited by various protonophores, and polyamines accumulated in membrane vesicles when D-lactate was added as an energy source or when a membrane potential was imposed artificially by the addition of valinomycin to K+-loaded vesicles. These results show that the uptake was dependent on proton motive force. Transported [14C]putrescine and [14C]spermidine were not excreted by intact cells upon the addition either of carbonyl cyanide m-chlorophenylhydrazone, A23187, and Ca2+ or of an excess amount of nonlabeled polyamine. However, they were excreted by membrane vesicles, although the degree of spermidine efflux was much lower than that of putrescine efflux. These results suggest that the apparent unidirectionality in intact cells has arisen from polyamine binding to nucleic acids, thus giving rise to a negligible free intracellular concentration of polyamines. Polyamine uptake, especially putrescine uptake, was inhibited strongly by monovalent cations. The Mg2+ ion inhibited spermidine and spermine uptake but not putrescine uptake.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger E. A., Heppel L. A. Different mechanisms of energy coupling for the shock-sensitive and shock-resistant amino acid permeases of Escherichia coli. J Biol Chem. 1974 Dec 25;249(24):7747–7755. [PubMed] [Google Scholar]
- Canellakis E. S., Viceps-Madore D., Kyriakidis D. A., Heller J. S. The regulation and function of ornithine decarboxylase and of the polyamines. Curr Top Cell Regul. 1979;15:155–202. [PubMed] [Google Scholar]
- Fujimoto S., Igarashi K., Shrestha R. D., Miyazaki M., Okui K. Antitumor effects of two polyamine antimetabolites combined with mitomycin C on human stomach cancer cells xenotransplanted into nude mice. Int J Cancer. 1985 Jun 15;35(6):821–825. doi: 10.1002/ijc.2910350620. [DOI] [PubMed] [Google Scholar]
- Hirata H., Altendorf K., Harold F. M. Energy coupling in membrane vesicles of Escherichia coli. I. Accumulation of metabolites in response to an electrical potential. J Biol Chem. 1974 May 10;249(9):2939–2945. [PubMed] [Google Scholar]
- Igarashi K., Kashiwagi K., Kishida K., Watanabe Y., Kogo A., Hirose S. Defect in the split proteins of 30-S ribosomal subunits and under-methylation of 16-S ribosomal RNA in a polyamine-requiring mutant of Escherichia coli grown in the absence of polyamines. Eur J Biochem. 1979 Jan 15;93(2):345–353. doi: 10.1111/j.1432-1033.1979.tb12829.x. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Mitsui K., Kubota M., Shirakuma M., Ohnishi R., Hirose S. Effect of polyamines on synthesis and degradation of guanosine 5'-diphosphate 3'-diphosphate. Biochim Biophys Acta. 1983 Feb 22;755(3):326–331. doi: 10.1016/0304-4165(83)90234-9. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Porter C. W., Morris D. R. Comparison of specificity of inhibition of polyamine synthesis in bovine lymphocytes by ethylglyoxal bis(guanylhydrazone) and methylglyoxal bis(guanylhydrazone). Cancer Res. 1984 Nov;44(11):5326–5331. [PubMed] [Google Scholar]
- Igarashi K., Sakamoto I., Goto N., Kashiwagi K., Honma R., Hirose S. Interaction between polyamines and nucleic acids or phospholipids. Arch Biochem Biophys. 1982 Dec;219(2):438–443. doi: 10.1016/0003-9861(82)90175-8. [DOI] [PubMed] [Google Scholar]
- Kimmich G. A., Randles J., Brand J. S. Assay of picomole amounts of ATP, ADP, and AMP using the luciferase enzyme system. Anal Biochem. 1975 Nov;69(1):187–206. doi: 10.1016/0003-2697(75)90580-1. [DOI] [PubMed] [Google Scholar]
- Kumagai H., Igarashi K., Yoshikawa M., Hirose S. Effects of polyamines on the activities of Escherichia coli ribonuclease I and II. J Biochem. 1977 Feb;81(2):381–388. doi: 10.1093/oxfordjournals.jbchem.a131469. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linderoth N., Morris D. R. Structural specificity of the triamines sym-homospermidine and aminopropylcadaverine in stimulating growth of spermidine auxotrophs of Escherichia coli. Biochem Biophys Res Commun. 1983 Dec 16;117(2):616–622. doi: 10.1016/0006-291x(83)91245-7. [DOI] [PubMed] [Google Scholar]
- MORRISON W. R. A FAST, SIMPLE AND RELIABLE METHOD FOR THE MICRODETERMINATION OF PHOSPHORUS IN BIOLOGICAL MATERIALS. Anal Biochem. 1964 Feb;7:218–224. doi: 10.1016/0003-2697(64)90231-3. [DOI] [PubMed] [Google Scholar]
- Munro G. F., Bell C. A., Lederman M. Multiple transport components for putrescine in Escherichia coli. J Bacteriol. 1974 Jun;118(3):952–963. doi: 10.1128/jb.118.3.952-963.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter C. W., Miller J., Bergeron R. J. Aliphatic chain length specificity of the polyamine transport system in ascites L1210 leukemia cells. Cancer Res. 1984 Jan;44(1):126–128. [PubMed] [Google Scholar]
- Rinehart C. A., Jr, Chen K. Y. Characterization of the polyamine transport system in mouse neuroblastoma cells. Effects of sodium and system A amino acids. J Biol Chem. 1984 Apr 25;259(8):4750–4756. [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Methionine adenosyltransferase (S-adenosylmethionine synthetase) and S-adenosylmethionine decarboxylase. Adv Enzymol Relat Areas Mol Biol. 1984;56:251–282. doi: 10.1002/9780470123027.ch4. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines in microorganisms. Microbiol Rev. 1985 Mar;49(1):81–99. doi: 10.1128/mr.49.1.81-99.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Transport systems for 1,4-diaminobutane, spermidine, and spermine in Escherichia coli. J Biol Chem. 1966 Aug 25;241(16):3714–3723. [PubMed] [Google Scholar]



