Abstract
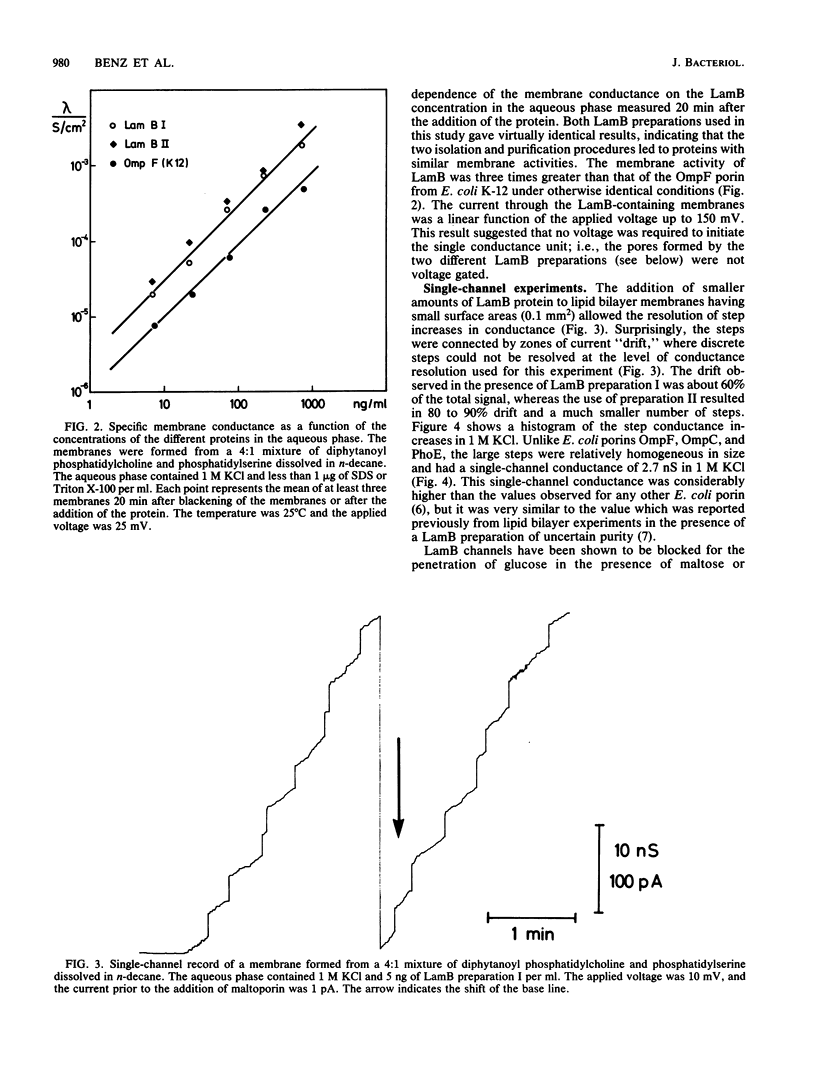
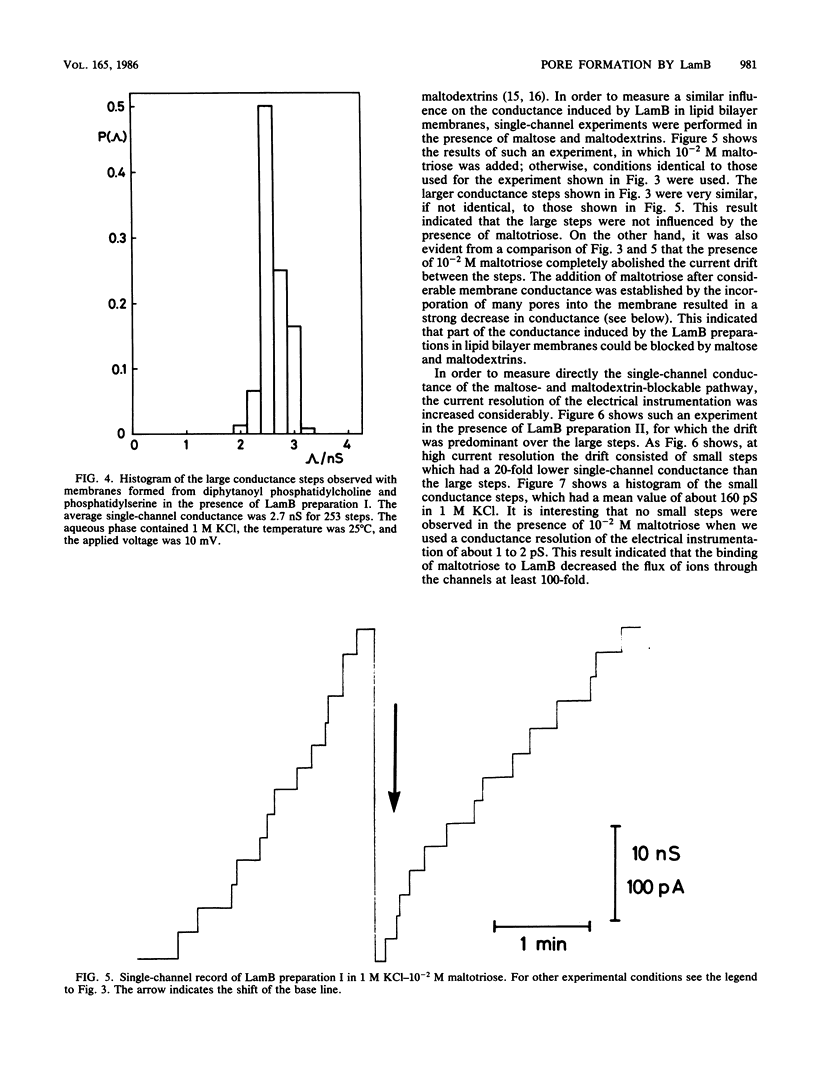
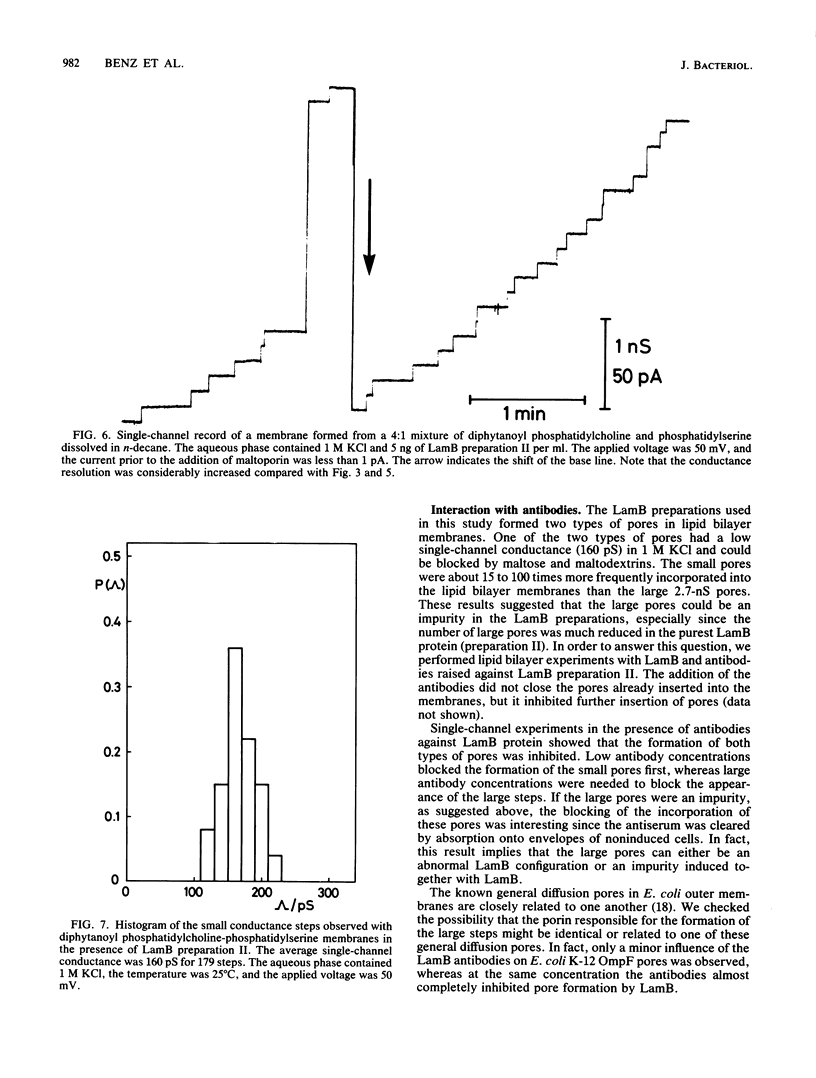
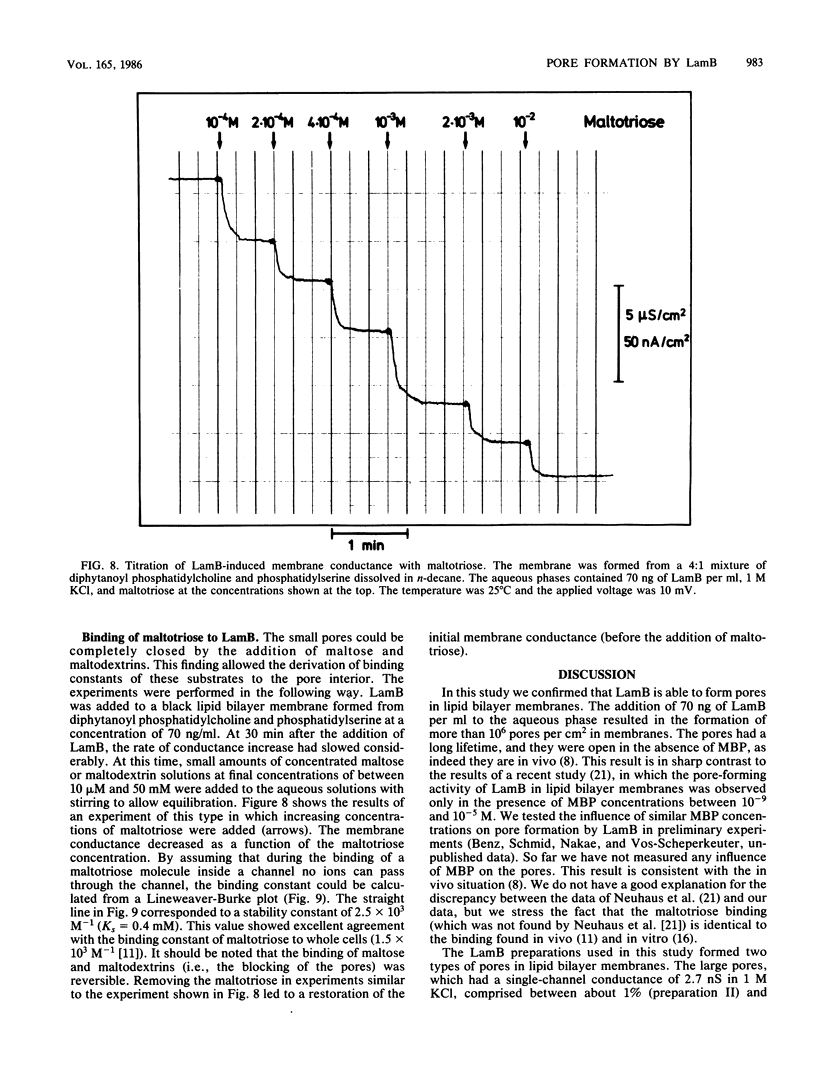
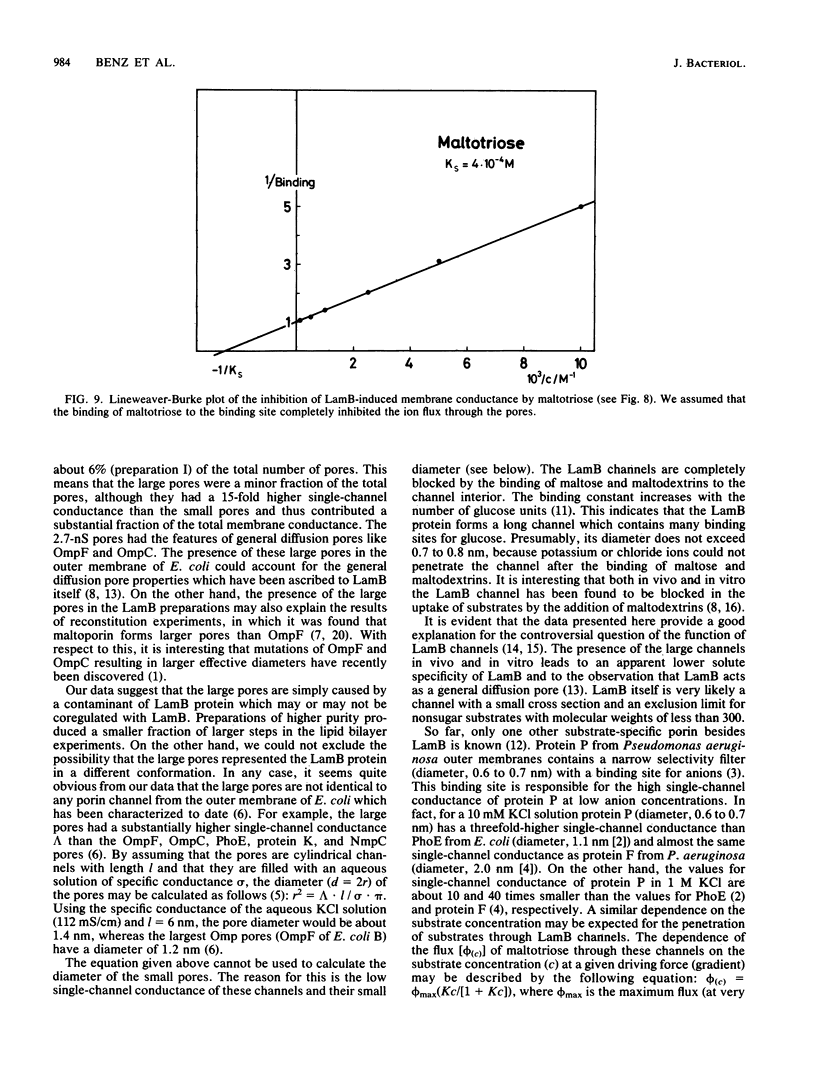
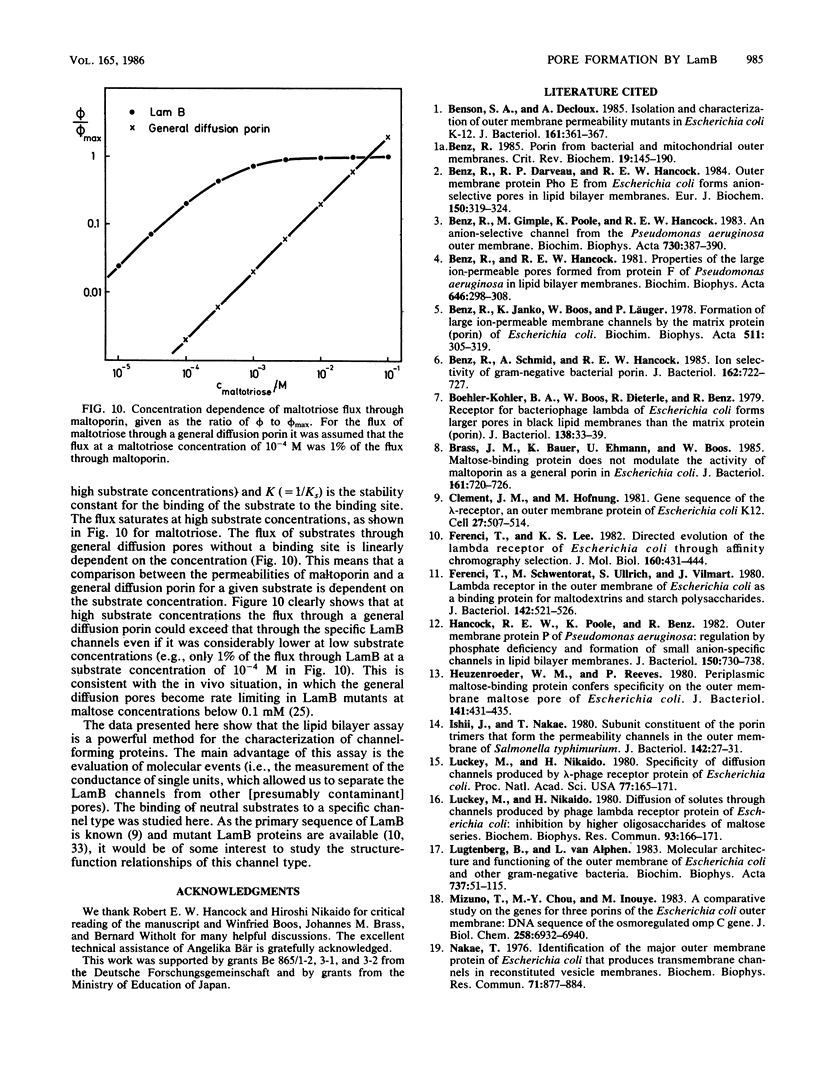
Lipid bilayer experiments were performed in the presence of different Escherichia coli LamB preparations. These LamB preparations formed two types of pores in the membranes. Large pores, which had a single-channel conductance of 2.7 nS and comprised about 1 to 6% of the total pores, were presumably contaminants which might have been induced together with LamB. LamB itself formed small pores with a single-channel conductance of 160 pS in 1 M KCl. These pores could be completely blocked by the addition of maltose and maltodextrins. Titration of the pore conductance with maltotriose suggested that there was a binding site inside the pores with a Ks of 2.5 X 10(-4) M for maltotriose. On the basis of our data we concluded that the structure of the LamB channels is quite different from the structures of the channels of general diffusion porins, such as OmpF and OmpC.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson S. A., Decloux A. Isolation and characterization of outer membrane permeability mutants in Escherichia coli K-12. J Bacteriol. 1985 Jan;161(1):361–367. doi: 10.1128/jb.161.1.361-367.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R., Darveau R. P., Hancock R. E. Outer-membrane protein PhoE from Escherichia coli forms anion-selective pores in lipid-bilayer membranes. Eur J Biochem. 1984 Apr 16;140(2):319–324. doi: 10.1111/j.1432-1033.1984.tb08104.x. [DOI] [PubMed] [Google Scholar]
- Benz R., Hancock R. E. Properties of the large ion-permeable pores formed from protein F of Pseudomonas aeruginosa in lipid bilayer membranes. Biochim Biophys Acta. 1981 Aug 20;646(2):298–308. doi: 10.1016/0005-2736(81)90336-9. [DOI] [PubMed] [Google Scholar]
- Benz R., Janko K., Boos W., Läuger P. Formation of large, ion-permeable membrane channels by the matrix protein (porin) of Escherichia coli. Biochim Biophys Acta. 1978 Aug 17;511(3):305–319. doi: 10.1016/0005-2736(78)90269-9. [DOI] [PubMed] [Google Scholar]
- Benz R. Porin from bacterial and mitochondrial outer membranes. CRC Crit Rev Biochem. 1985;19(2):145–190. doi: 10.3109/10409238509082542. [DOI] [PubMed] [Google Scholar]
- Benz R., Schmid A., Hancock R. E. Ion selectivity of gram-negative bacterial porins. J Bacteriol. 1985 May;162(2):722–727. doi: 10.1128/jb.162.2.722-727.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehler-Kohler B. A., Boos W., Dieterle R., Benz R. Receptor for bacteriophage lambda of Escherichia coli forms larger pores in black lipid membranes than the matrix protein (porin). J Bacteriol. 1979 Apr;138(1):33–39. doi: 10.1128/jb.138.1.33-39.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass J. M., Bauer K., Ehmann U., Boos W. Maltose-binding protein does not modulate the activity of maltoporin as a general porin in Escherichia coli. J Bacteriol. 1985 Feb;161(2):720–726. doi: 10.1128/jb.161.2.720-726.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clément J. M., Hofnung M. Gene sequence of the lambda receptor, an outer membrane protein of E. coli K12. Cell. 1981 Dec;27(3 Pt 2):507–514. doi: 10.1016/0092-8674(81)90392-5. [DOI] [PubMed] [Google Scholar]
- Ferenci T., Lee K. S. Directed evolution of the lambda receptor of Escherichia coli through affinity chromatographic selection. J Mol Biol. 1982 Sep 25;160(3):431–444. doi: 10.1016/0022-2836(82)90306-0. [DOI] [PubMed] [Google Scholar]
- Ferenci T., Schwentorat M., Ullrich S., Vilmart J. Lambda receptor in the outer membrane of Escherichia coli as a binding protein for maltodextrins and starch polysaccharides. J Bacteriol. 1980 May;142(2):521–526. doi: 10.1128/jb.142.2.521-526.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Poole K., Benz R. Outer membrane protein P of Pseudomonas aeruginosa: regulation by phosphate deficiency and formation of small anion-specific channels in lipid bilayer membranes. J Bacteriol. 1982 May;150(2):730–738. doi: 10.1128/jb.150.2.730-738.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuzenroeder M. W., Reeves P. Periplasmic maltose-binding protein confers specificity on the outer membrane maltose pore of Escherichia coli. J Bacteriol. 1980 Feb;141(2):431–435. doi: 10.1128/jb.141.2.431-435.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii J., Nakae T. Subunit constituent of the porin trimers that form the permeability channels in the outer membrane of Salmonella typhimurium. J Bacteriol. 1980 Apr;142(1):27–31. doi: 10.1128/jb.142.1.27-31.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey M., Nikaido H. Diffusion of solutes through channels produced by phage lambda receptor protein of Escherichia coli: inhibition by higher oligosaccharides of maltose series. Biochem Biophys Res Commun. 1980 Mar 13;93(1):166–171. doi: 10.1016/s0006-291x(80)80261-0. [DOI] [PubMed] [Google Scholar]
- Luckey M., Nikaido H. Specificity of diffusion channels produced by lambda phage receptor protein of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):167–171. doi: 10.1073/pnas.77.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Chou M. Y., Inouye M. A comparative study on the genes for three porins of the Escherichia coli outer membrane. DNA sequence of the osmoregulated ompC gene. J Biol Chem. 1983 Jun 10;258(11):6932–6940. [PubMed] [Google Scholar]
- Nakae T. A porin activity of purified lambda-receptor protein from Escherichia coli in reconstituted vesicle membranes. Biochem Biophys Res Commun. 1979 Jun 13;88(3):774–781. doi: 10.1016/0006-291x(79)91475-x. [DOI] [PubMed] [Google Scholar]
- Nakae T. Identification of the outer membrane protein of E. coli that produces transmembrane channels in reconstituted vesicle membranes. Biochem Biophys Res Commun. 1976 Aug 9;71(3):877–884. doi: 10.1016/0006-291x(76)90913-x. [DOI] [PubMed] [Google Scholar]
- Neuhaus J. M., Schindler H., Rosenbusch J. P. The periplasmic maltose-binding protein modifies the channel-forming characteristics of maltoporin. EMBO J. 1983;2(11):1987–1991. doi: 10.1002/j.1460-2075.1983.tb01689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y. Porin channels in Escherichia coli: studies with liposomes reconstituted from purified proteins. J Bacteriol. 1983 Jan;153(1):241–252. doi: 10.1128/jb.153.1.241-252.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva E. T. Major outer membrane protein in Salmonella typhimurium induced by maltose. J Bacteriol. 1978 Oct;136(1):286–294. doi: 10.1128/jb.136.1.286-294.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmelcman S., Hofnung M. Maltose transport in Escherichia coli K-12: involvement of the bacteriophage lambda receptor. J Bacteriol. 1975 Oct;124(1):112–118. doi: 10.1128/jb.124.1.112-118.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmelcman S., Schwartz M., Silhavy T. J., Boos W. Maltose transport in Escherichia coli K12. A comparison of transport kinetics in wild-type and lambda-resistant mutants as measured by fluorescence quenching. Eur J Biochem. 1976 May 17;65(1):13–19. doi: 10.1111/j.1432-1033.1976.tb10383.x. [DOI] [PubMed] [Google Scholar]
- Tokunaga M., Tokunaga H., Nakae T. The outer membrane permeability of Gram-negative bacteria: determination of permeability rate in reconstituted vesicle membranes. FEBS Lett. 1979 Oct 1;106(1):85–88. doi: 10.1016/0014-5793(79)80700-0. [DOI] [PubMed] [Google Scholar]
- Tokunaga M., Tokunaga H., Okajima Y., Nakae T. Characterization of porins from the outer membrane of Salmonella typhimurium. 2. Physical properties of the functional oligomeric aggregates. Eur J Biochem. 1979 Apr;95(3):441–448. doi: 10.1111/j.1432-1033.1979.tb12983.x. [DOI] [PubMed] [Google Scholar]
- Tommassen J., Lugtenberg B. Outer membrane protein e of Escherichia coli K-12 is co-regulated with alkaline phosphatase. J Bacteriol. 1980 Jul;143(1):151–157. doi: 10.1128/jb.143.1.151-157.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos-Scheperkeuter G. H., Hofnung M., Witholt B. High-sensitivity detection of newly induced LamB protein on the Escherichia coli cell surface. J Bacteriol. 1984 Aug;159(2):435–439. doi: 10.1128/jb.159.2.435-439.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos-Scheperkeuter G. H., Witholt B. Assembly pathway of newly synthesized LamB protein an outer membrane protein of Escherichia coli K-12. J Mol Biol. 1984 Jun 5;175(4):511–528. doi: 10.1016/0022-2836(84)90182-7. [DOI] [PubMed] [Google Scholar]
- Wandersman C., Schwartz M. Mutations that alter the transport function of the LamB protein in Escherichia coli. J Bacteriol. 1982 Jul;151(1):15–21. doi: 10.1128/jb.151.1.15-21.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Meyenburg K., Nikaido H. Outer membrane of gram-negative bacteria. XVII. Secificity of transport process catalyzed by the lambda-receptor protein in Escherichia coli. Biochem Biophys Res Commun. 1977 Oct 10;78(3):1100–1107. doi: 10.1016/0006-291x(77)90534-4. [DOI] [PubMed] [Google Scholar]