Abstract
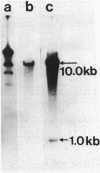
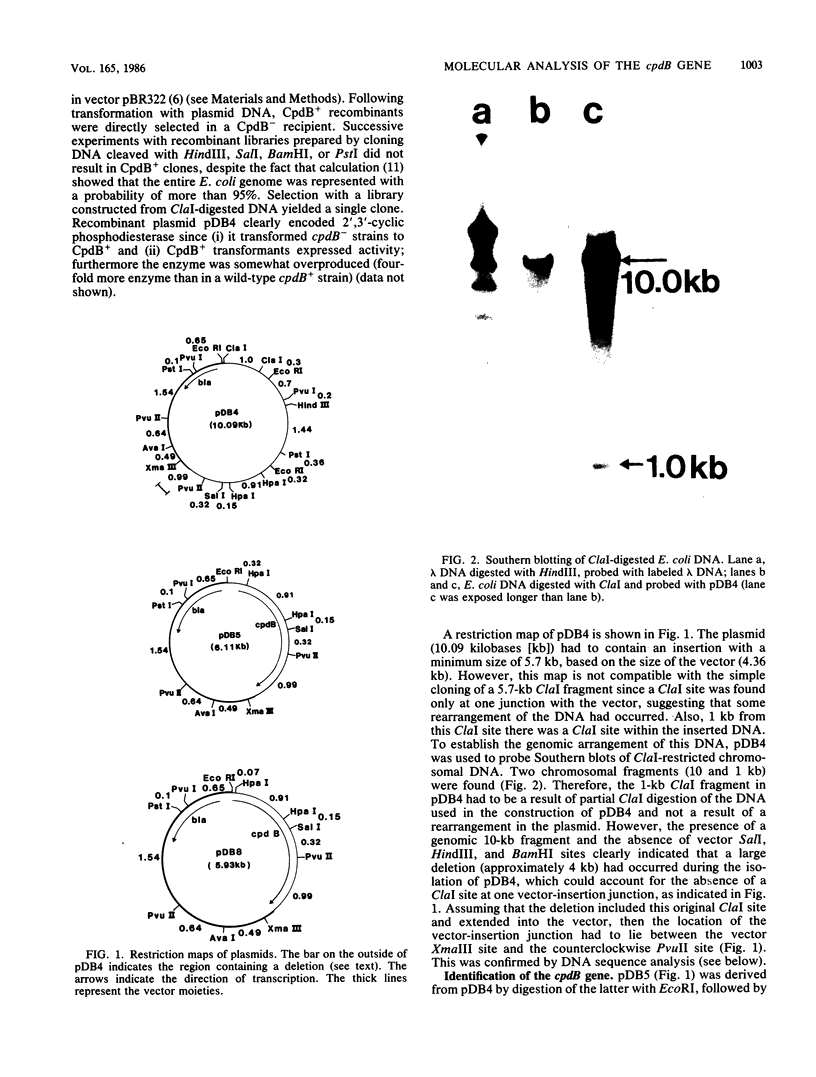
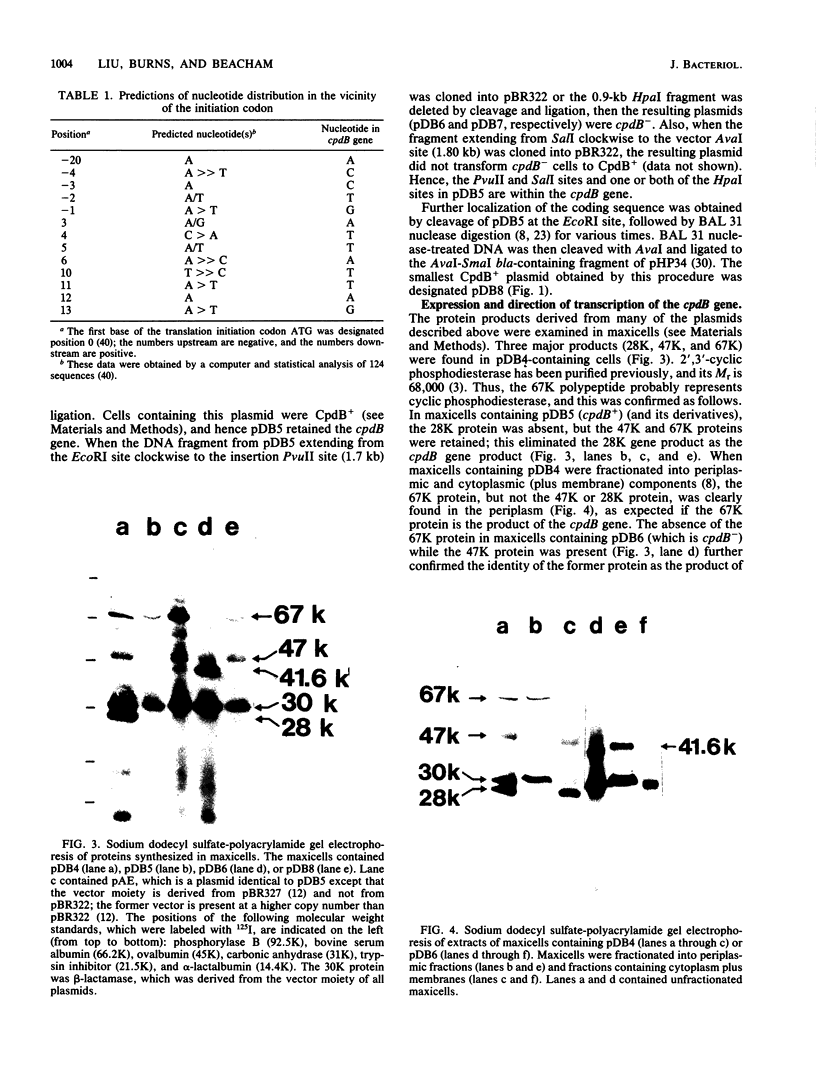
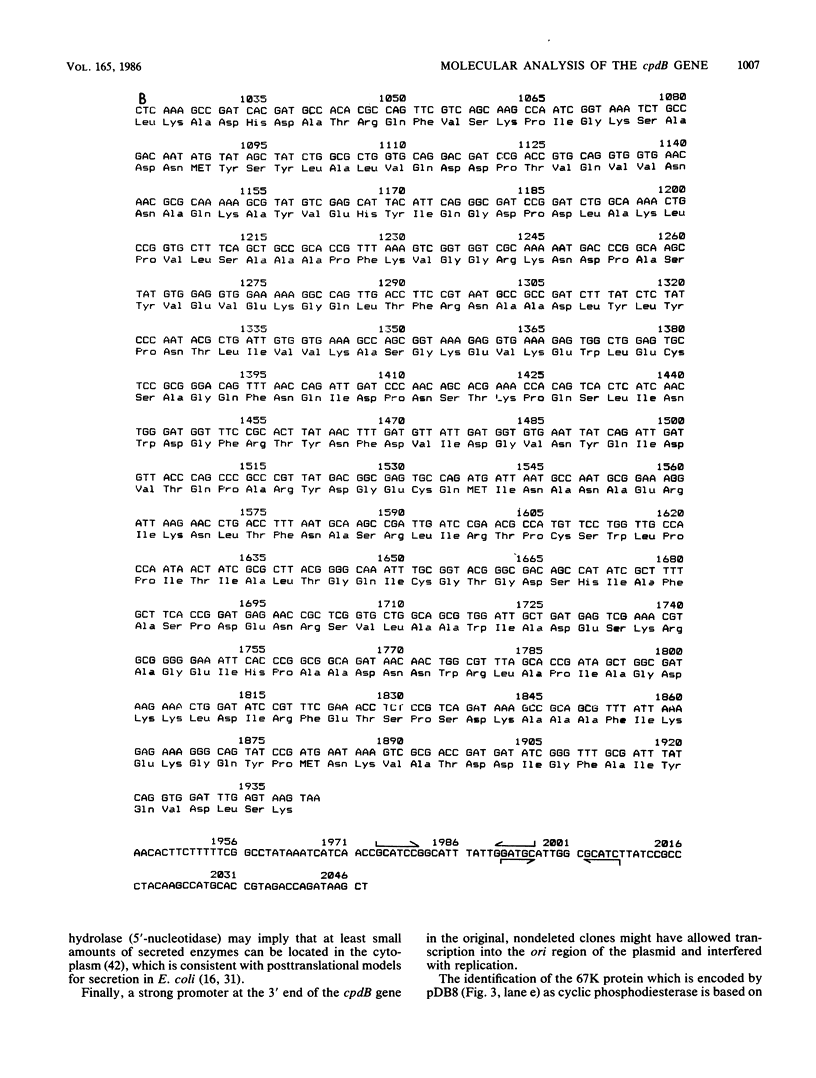
The cpdB gene encodes a periplasmic 2',3'-cyclic phosphodiesterase (3'-nucleotidase). This enzyme has been purified previously and the gene is located at 96 min on the Escherichia coli chromosome. In this study the cpdB gene was cloned from ClaI-cleaved DNA, and the gene product was identified. DNA blotting experiments showed that the recombinant plasmid contains a deletion with respect to the expected genomic fragment of approximately 4 kilobases, which extends into the vector. Furthermore, the gene was absent from three other recombinant libraries. Together, these findings suggest the presence in the genome of an adjacent gene whose product is lethal when it is present on a multicopy plasmid. The nucleotide sequence of the cpdB gene was also determined. The 5' and 3' untranslated sequences contain characteristic sequences that are involved in the initiation and termination of transcription, including two possible promoters, one of which may contain two overlapping -10 sequences. A strong Shine-Dalgarno sequence is followed by an open reading frame which corresponds to a protein having a molecular weight of 70,954. The first 19 amino acid residues have the characteristics of a signal peptide. The 3' untranslated sequence contains two putative rho-independent transcription terminators having low thermodynamic stability.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANRAKU Y. A NEW CYCLIC PHOSPHODIESTERASE HAVING A 3'-NUCLEOTIDASE ACTIVITY FROM ESCHERICHIA COLI B. I. PURIFICATION AND SOME PROPERTIES OF THE ENZYME. J Biol Chem. 1964 Oct;239:3412–3419. [PubMed] [Google Scholar]
- ANRAKU Y. A NEW CYCLIC PHOSPHODIESTERASE HAVING A 3'-NUCLEOTIDASE ACTIVITY FROM ESCHERICHIA COLI B. II. FURTHER STUDIES ON SUBSTRATE SPECIFICITY AND MODE OF ACTION OF THE ENZYME. J Biol Chem. 1964 Oct;239:3420–3424. [PubMed] [Google Scholar]
- Beacham I. R., Garrett S. Isolation of Escherichia coli mutants (cpdB) deficient in periplasmic 2':3'-cyclic phosphodiesterase and genetic mapping of the cpdB locus. J Gen Microbiol. 1980 Jul;119(1):31–34. doi: 10.1099/00221287-119-1-31. [DOI] [PubMed] [Google Scholar]
- Beacham I. R. Periplasmic enzymes in gram-negative bacteria. Int J Biochem. 1979;10(11):877–883. doi: 10.1016/0020-711x(79)90117-4. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Brendel V., Trifonov E. N. A computer algorithm for testing potential prokaryotic terminators. Nucleic Acids Res. 1984 May 25;12(10):4411–4427. doi: 10.1093/nar/12.10.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns D. M., Abraham L. J., Beacham I. R. Characterization of the ush gene of Escherichia coli and its protein products. Gene. 1983 Nov;25(2-3):343–353. doi: 10.1016/0378-1119(83)90239-1. [DOI] [PubMed] [Google Scholar]
- Burns D. M., Beacham I. R. A method for the ligation of DNA following isolation from low melting temperature agarose. Anal Biochem. 1983 Nov;135(1):48–51. doi: 10.1016/0003-2697(83)90728-5. [DOI] [PubMed] [Google Scholar]
- Burns D. M., Beacham I. R. Positive selection vectors: a small plasmid vector useful for the direct selection of Sau3A-generated overlapping DNA fragments. Gene. 1984 Mar;27(3):323–325. doi: 10.1016/0378-1119(84)90077-5. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Covarrubias L., Cervantes L., Covarrubias A., Soberón X., Vichido I., Blanco A., Kupersztoch-Portnoy Y. M., Bolivar F. Construction and characterization of new cloning vehicles. V. Mobilization and coding properties of pBR322 and several deletion derivatives including pBR327 and pBR328. Gene. 1981 Jan-Feb;13(1):25–35. doi: 10.1016/0378-1119(81)90040-8. [DOI] [PubMed] [Google Scholar]
- Cowman A., Beacham I. R. Molecular cloning of the gene (ush) from Escherichia coli specifying periplasmic UDP-sugar hydrolase (5'-nucleotidase). Gene. 1980 Dec;12(3-4):281–286. doi: 10.1016/0378-1119(80)90111-0. [DOI] [PubMed] [Google Scholar]
- Crowlesmith I., Gamon K. Rate of translation and kinetics of processing of newly synthesized molecules of two major outer-membrane proteins, the OmpA and OmpF proteins, of Escherichia coli K12. Eur J Biochem. 1982 Jun;124(3):577–583. doi: 10.1111/j.1432-1033.1982.tb06633.x. [DOI] [PubMed] [Google Scholar]
- Dalbadie-McFarland G., Cohen L. W., Riggs A. D., Morin C., Itakura K., Richards J. H. Oligonucleotide-directed mutagenesis as a general and powerful method for studies of protein function. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6409–6413. doi: 10.1073/pnas.79.21.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garwin J. L., Beckwith J. Secretion and processing of ribose-binding protein in Escherichia coli. J Bacteriol. 1982 Feb;149(2):789–792. doi: 10.1128/jb.149.2.789-792.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser L., Melo A., Paul R. Uridine diphosphate sugar hydrolase. Purification of enzyme and protein inhibitor. J Biol Chem. 1967 Apr 25;242(8):1944–1954. [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemura T., Ozeki H. Codon usage and transfer RNA contents: organism-specific codon-choice patterns in reference to the isoacceptor contents. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):1087–1097. doi: 10.1101/sqb.1983.047.01.123. [DOI] [PubMed] [Google Scholar]
- Inouye M., Halegoua S. Secretion and membrane localization of proteins in Escherichia coli. CRC Crit Rev Biochem. 1980;7(4):339–371. doi: 10.3109/10409238009105465. [DOI] [PubMed] [Google Scholar]
- Inouye S., Soberon X., Franceschini T., Nakamura K., Itakura K., Inouye M. Role of positive charge on the amino-terminal region of the signal peptide in protein secretion across the membrane. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3438–3441. doi: 10.1073/pnas.79.11.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legerski R. J., Hodnett J. L., Gray H. B., Jr Extracellular nucleases of pseudomonas BAL 31. III. Use of the double-strand deoxyriboexonuclease activity as the basis of a convenient method for the mapping of fragments of DNA produced by cleavage with restriction enzymes. Nucleic Acids Res. 1978 May;5(5):1445–1464. doi: 10.1093/nar/5.5.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magota K., Otsuji N., Miki T., Horiuchi T., Tsunasawa S., Kondo J., Sakiyama F., Amemura M., Morita T., Shinagawa H. Nucleotide sequence of the phoS gene, the structural gene for the phosphate-binding protein of Escherichia coli. J Bacteriol. 1984 Mar;157(3):909–917. doi: 10.1128/jb.157.3.909-917.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Neu H. C. The 5'-nucleotidase of Escherichia coli. II. Surface localization and purification of the Escherichia coli 5'-nucleotidase inhibitor. J Biol Chem. 1967 Sep 10;242(17):3905–3911. [PubMed] [Google Scholar]
- Ohmori H., Kimura M., Nagata T., Sakakibara Y. Structural analysis of the dnaA and dnaN genes of Escherichia coli. Gene. 1984 May;28(2):159–170. doi: 10.1016/0378-1119(84)90253-1. [DOI] [PubMed] [Google Scholar]
- Platt T. Termination of transcription and its regulation in the tryptophan operon of E. coli. Cell. 1981 Apr;24(1):10–23. doi: 10.1016/0092-8674(81)90496-7. [DOI] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. A modified pBR322 vector with improved properties for the cloning, recovery, and sequencing of blunt-ended DNA fragments. Gene. 1982 Feb;17(2):189–196. doi: 10.1016/0378-1119(82)90072-5. [DOI] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J. Export of protein in bacteria. Microbiol Rev. 1984 Dec;48(4):290–298. doi: 10.1128/mr.48.4.290-298.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy T. J., Benson S. A., Emr S. D. Mechanisms of protein localization. Microbiol Rev. 1983 Sep;47(3):313–344. doi: 10.1128/mr.47.3.313-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slocombe P., Easton A., Boseley P., Burke D. C. High-level expression of an interferon alpha 2 gene cloned in phage M13mp7 and subsequent purification with a monoclonal antibody. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5455–5459. doi: 10.1073/pnas.79.18.5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. R., Calvo J. M. Nucleotide sequence of the E coli gene coding for dihydrofolate reductase. Nucleic Acids Res. 1980 May 24;8(10):2255–2274. doi: 10.1093/nar/8.10.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stoker N. G., Fairweather N. F., Spratt B. G. Versatile low-copy-number plasmid vectors for cloning in Escherichia coli. Gene. 1982 Jun;18(3):335–341. doi: 10.1016/0378-1119(82)90172-x. [DOI] [PubMed] [Google Scholar]
- Stormo G. D., Schneider T. D., Gold L. M. Characterization of translational initiation sites in E. coli. Nucleic Acids Res. 1982 May 11;10(9):2971–2996. doi: 10.1093/nar/10.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Taylor N. S., Beacham I. R. Synthesis and localisation of Escherichia coli UDP-glucose hydrolase (5'-nucleotidase), and demonstration of a cytoplasmic inhibitor of this enzyme in Salmonella typhimurium. Biochim Biophys Acta. 1975 Dec 5;411(2):216–221. doi: 10.1016/0304-4165(75)90301-3. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Vlasuk G. P., Inouye S., Ito H., Itakura K., Inouye M. Effects of the complete removal of basic amino acid residues from the signal peptide on secretion of lipoprotein in Escherichia coli. J Biol Chem. 1983 Jun 10;258(11):7141–7148. [PubMed] [Google Scholar]
- Watson M. E. Compilation of published signal sequences. Nucleic Acids Res. 1984 Jul 11;12(13):5145–5164. doi: 10.1093/nar/12.13.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. How signal sequences maintain cleavage specificity. J Mol Biol. 1984 Feb 25;173(2):243–251. doi: 10.1016/0022-2836(84)90192-x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. On the hydrophobic nature of signal sequences. Eur J Biochem. 1981 May 15;116(2):419–422. doi: 10.1111/j.1432-1033.1981.tb05351.x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]