Abstract
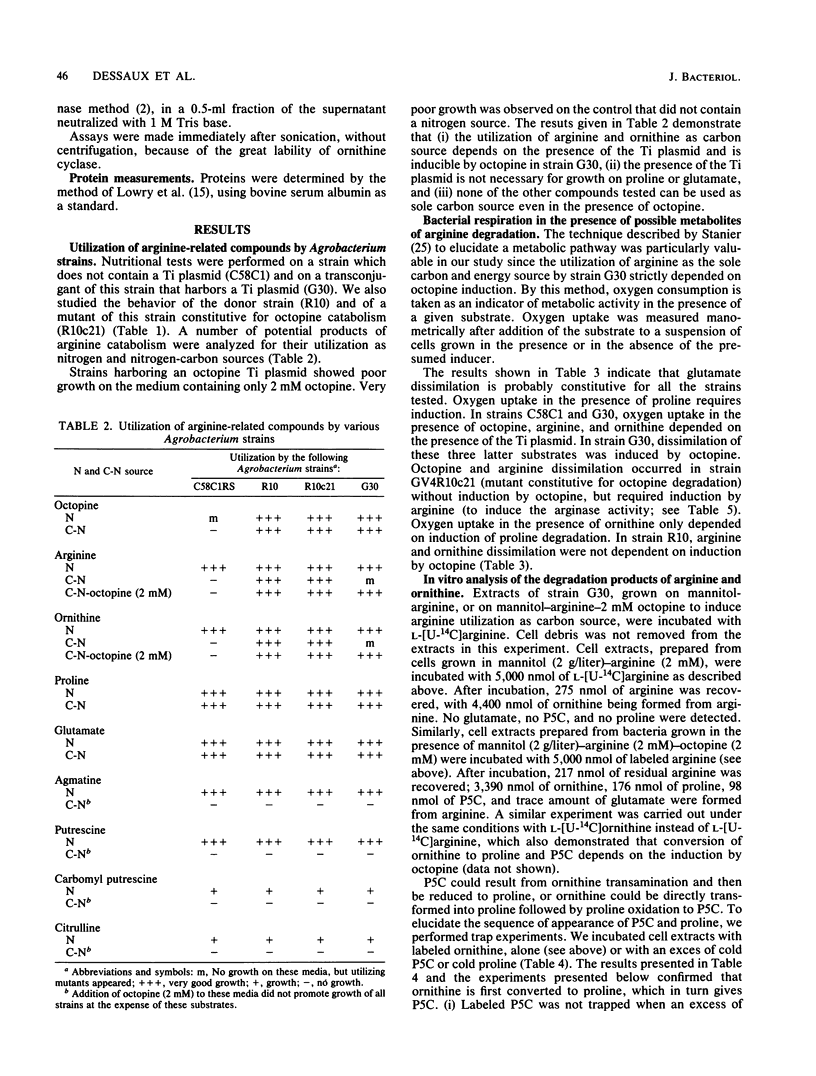
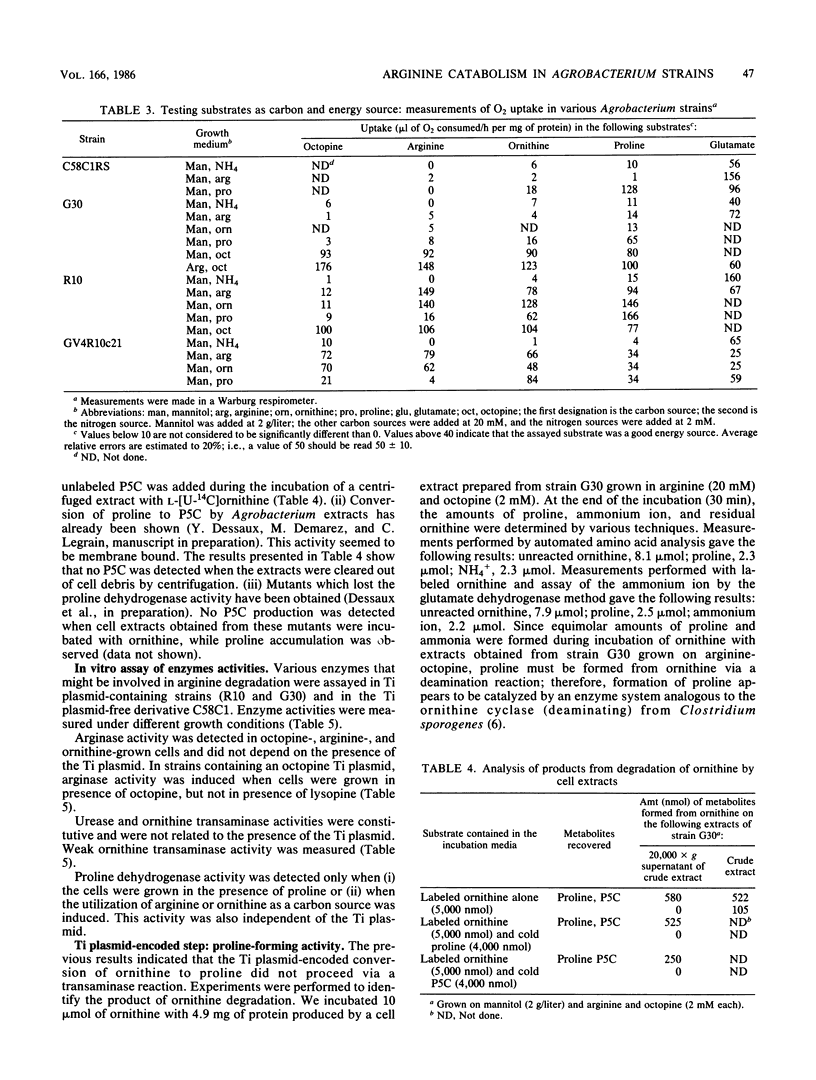
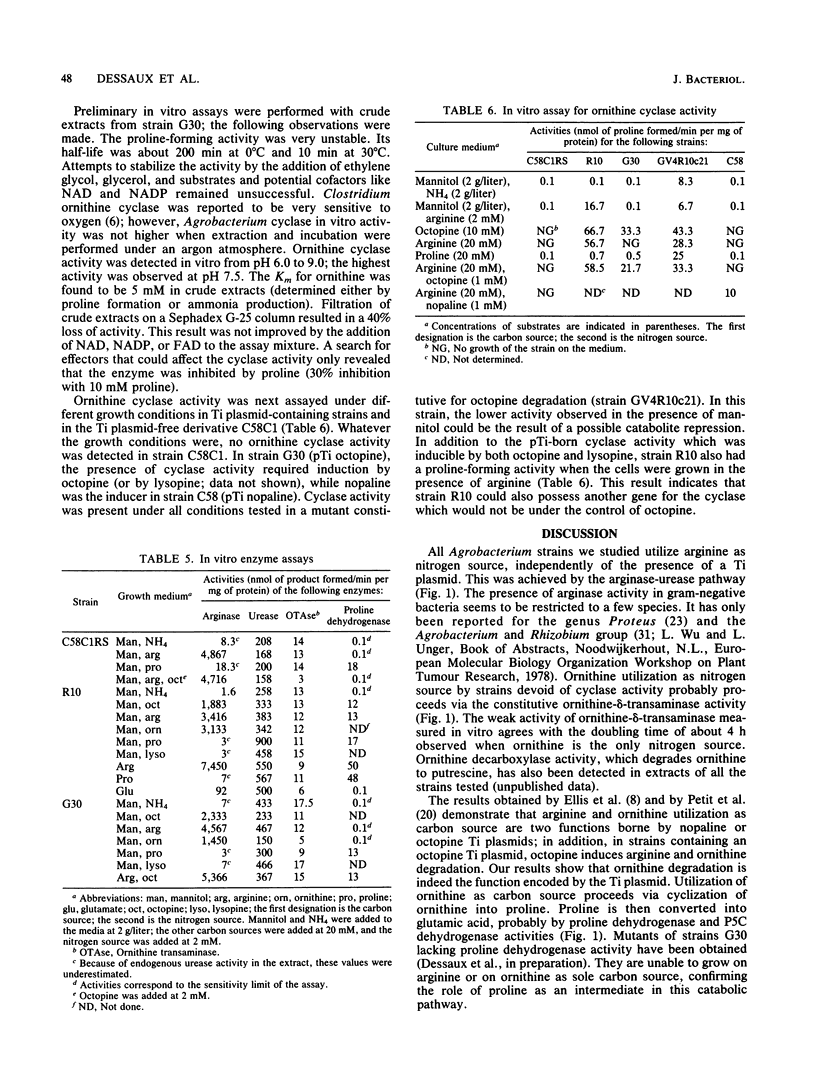
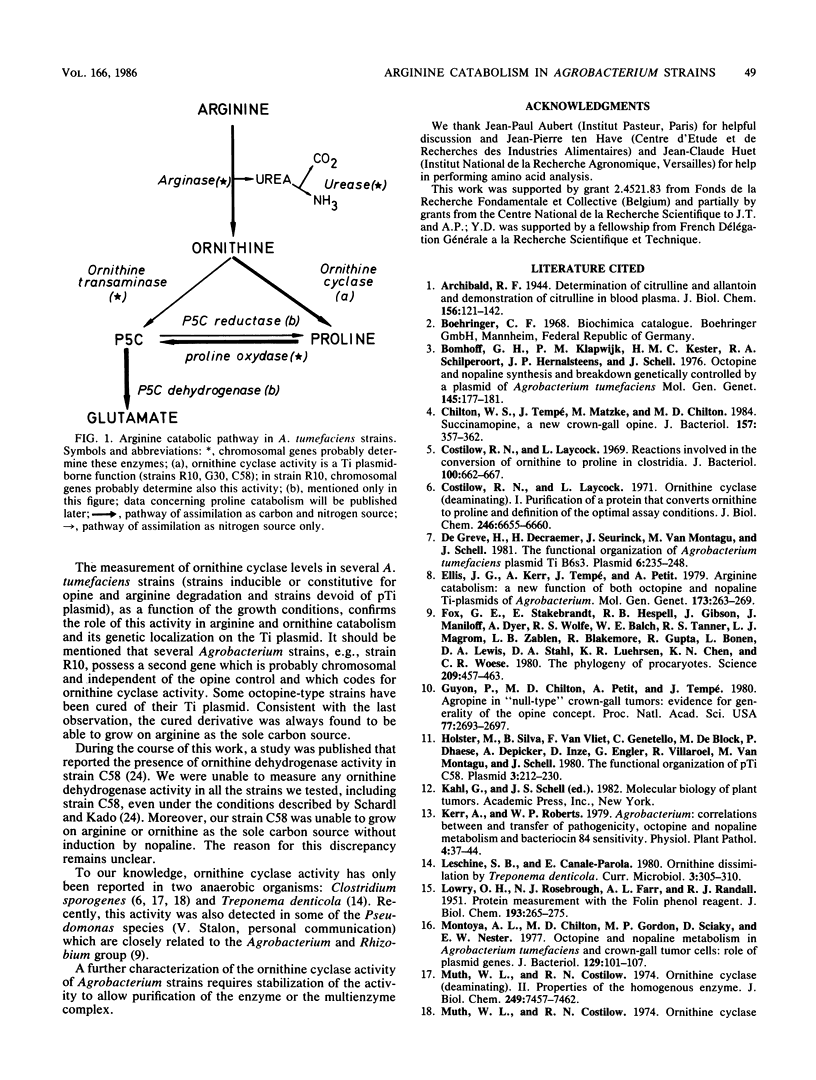
We present a study of the enzymatic activities involved in the pathway for arginine catabolism by Agrobacterium tumefaciens. Nitrogen from arginine is recovered through the arginase-urease pathway; the genes for these two activities are probably chromosomally born. Arginase was found to be inducible during growth in the presence of arginine or ornithine. Urease was constitutively expressed. Ornithine, resulting from the action of arginase on arginine, could be used as a nitrogen source via transamination to delta 1-pyrroline-5-carboxylate and reduction of the latter compound to proline by a reductase (both enzymatic activities are probably chromosomally encoded). Ornithine could also be used as a carbon source. Thus, we identified an ornithine cyclase activity that was responsible for direct conversion of ornithine to proline. This activity was found to be Ti plasmid encoded and inducible by growth in medium containing octopine or nopaline. The same activity was also chromosomally encoded in some Agrobacterium strains. In such strains, this activity was inducible during growth in arginine-containing medium.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bomhoff G., Klapwijk P. M., Kester H. C., Schilperoort R. A., Hernalsteens J. P., Schell J. Octopine and nopaline synthesis and breakdown genetically controlled by a plasmid of Agrobacterium tumefaciens. Mol Gen Genet. 1976 May 7;145(2):177–181. doi: 10.1007/BF00269591. [DOI] [PubMed] [Google Scholar]
- Chilton W. S., Tempé J., Matzke M., Chilton M. D. Succinamopine: a new crown gall opine. J Bacteriol. 1984 Feb;157(2):357–362. doi: 10.1128/jb.157.2.357-362.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costilow R. N., Laycock L. Ornithine cyclase (deaminating). Purification of a protein that converts ornithine to proline and definition of the optimal assay conditions. J Biol Chem. 1971 Nov;246(21):6655–6660. [PubMed] [Google Scholar]
- Costilow R. N., Laycock L. Reactions involved in the conversion of ornithine to proline in Clostridia. J Bacteriol. 1969 Nov;100(2):662–667. doi: 10.1128/jb.100.2.662-667.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Greve H., Decraemer H., Seurinck J., Van Montagu M., Schell J. The functional organization of the octopine Agrobacterium tumefaciens plasmid pTiB6s3. Plasmid. 1981 Sep;6(2):235–248. doi: 10.1016/0147-619x(81)90069-x. [DOI] [PubMed] [Google Scholar]
- Ellis J. G., Kerr A., Tempé J., Petit A. Arginine catabolism: a new function of both octopine and nopaline Ti-plasmids of Agrobacterium. Mol Gen Genet. 1979 Jun 20;173(3):263–269. doi: 10.1007/BF00268636. [DOI] [PubMed] [Google Scholar]
- Fox G. E., Stackebrandt E., Hespell R. B., Gibson J., Maniloff J., Dyer T. A., Wolfe R. S., Balch W. E., Tanner R. S., Magrum L. J. The phylogeny of prokaryotes. Science. 1980 Jul 25;209(4455):457–463. doi: 10.1126/science.6771870. [DOI] [PubMed] [Google Scholar]
- Guyon P., Chilton M. D., Petit A., Tempé J. Agropine in "null-type" crown gall tumors: Evidence for generality of the opine concept. Proc Natl Acad Sci U S A. 1980 May;77(5):2693–2697. doi: 10.1073/pnas.77.5.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsters M., Silva B., Van Vliet F., Genetello C., De Block M., Dhaese P., Depicker A., Inzé D., Engler G., Villarroel R. The functional organization of the nopaline A. tumefaciens plasmid pTiC58. Plasmid. 1980 Mar;3(2):212–230. doi: 10.1016/0147-619x(80)90110-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Montoya A. L., Chilton M. D., Gordon M. P., Sciaky D., Nester E. W. Octopine and nopaline metabolism in Agrobacterium tumefaciens and crown gall tumor cells: role of plasmid genes. J Bacteriol. 1977 Jan;129(1):101–107. doi: 10.1128/jb.129.1.101-107.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muth W. L., Costilow R. N. Ornithine cyclase (deaminating). II. Properties of the homogeneous enzyme. J Biol Chem. 1974 Dec 10;249(23):7457–7462. [PubMed] [Google Scholar]
- Prozesky O. W., Grabow W. O., van der Merwe S., Coetzee J. N. Arginine gene clusters in the Proteus-Providence group. J Gen Microbiol. 1973 Jul;77(1):237–240. doi: 10.1099/00221287-77-1-237. [DOI] [PubMed] [Google Scholar]
- Schardl C. L., Kado C. I. Ti plasmid and chromosomal ornithine catabolism genes of Agrobacterium tumefaciens C58. J Bacteriol. 1983 Jul;155(1):196–202. doi: 10.1128/jb.155.1.196-202.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y. Simultaneous Adaptation: A New Technique for the Study of Metabolic Pathways. J Bacteriol. 1947 Sep;54(3):339–348. doi: 10.1128/jb.54.3.339-348.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Larebeke N., Engler G., Holsters M., Van den Elsacker S., Zaenen I., Schilperoort R. A., Schell J. Large plasmid in Agrobacterium tumefaciens essential for crown gall-inducing ability. Nature. 1974 Nov 8;252(5479):169–170. doi: 10.1038/252169a0. [DOI] [PubMed] [Google Scholar]
- Van Larebeke N., Genetello C., Schell J., Schilperoort R. A., Hermans A. K., Van Montagu M., Hernalsteens J. P. Acquisition of tumour-inducing ability by non-oncogenic agrobacteria as a result of plasmid transfer. Nature. 1975 Jun 26;255(5511):742–743. doi: 10.1038/255742a0. [DOI] [PubMed] [Google Scholar]
- Watson B., Currier T. C., Gordon M. P., Chilton M. D., Nester E. W. Plasmid required for virulence of Agrobacterium tumefaciens. J Bacteriol. 1975 Jul;123(1):255–264. doi: 10.1128/jb.123.1.255-264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams I., Frank L. Improved chemical synthesis and enzymatic assay of delta-1-pyrroline-5-carboxylic acid. Anal Biochem. 1975 Mar;64(1):85–97. doi: 10.1016/0003-2697(75)90408-x. [DOI] [PubMed] [Google Scholar]



