Abstract
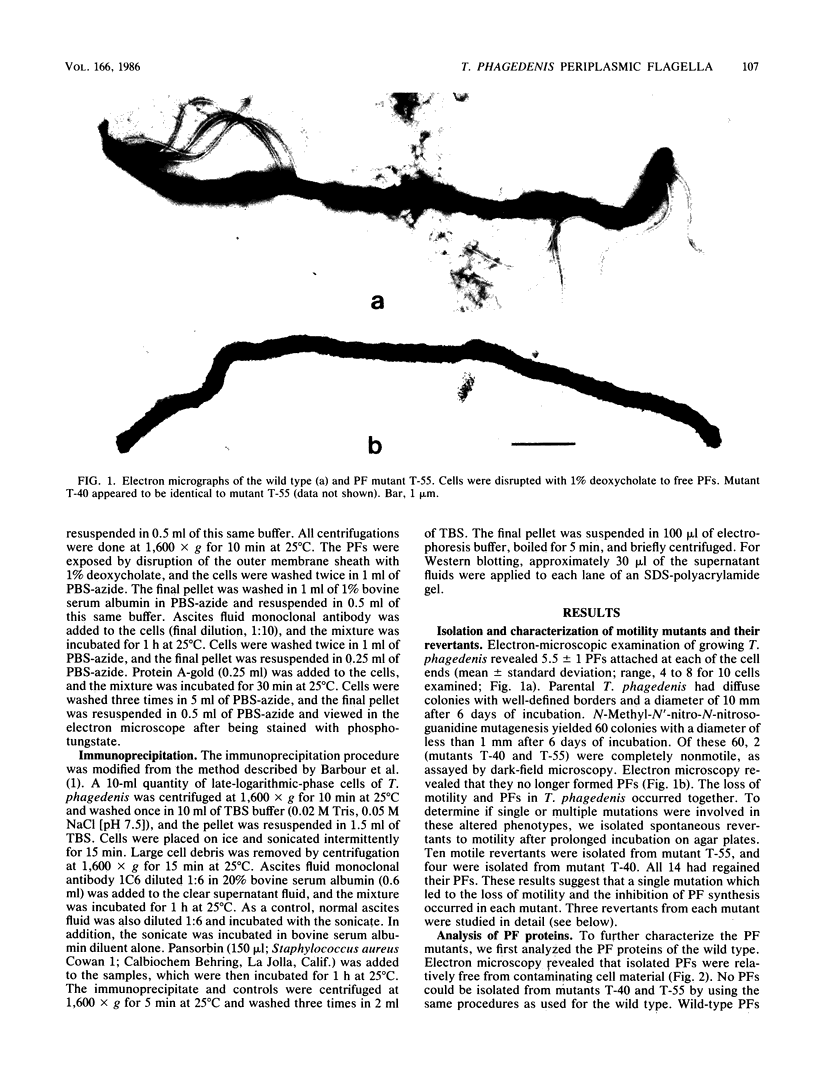
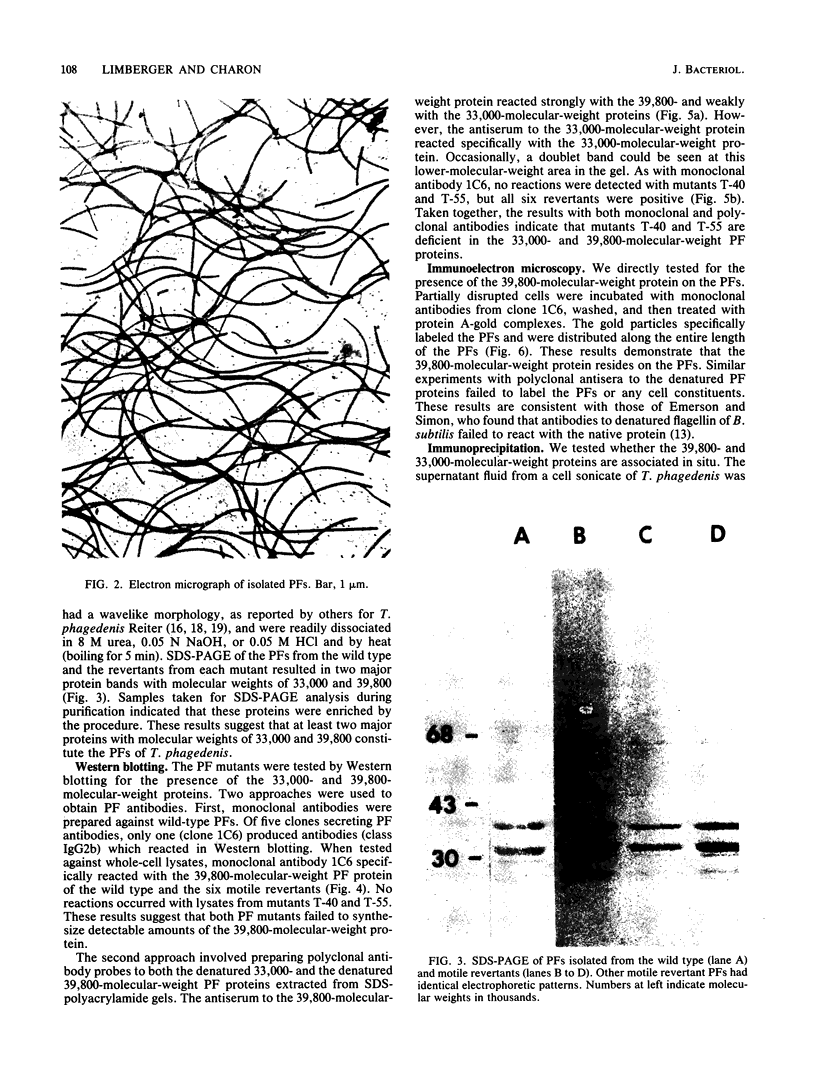
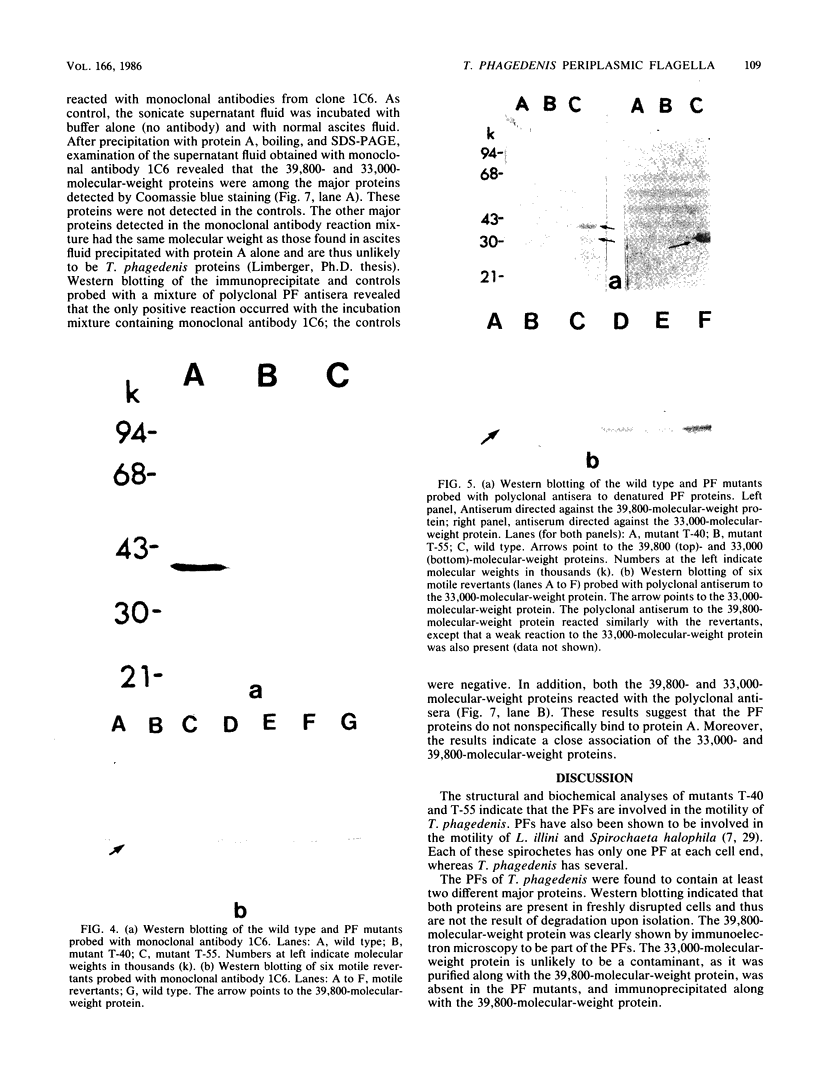
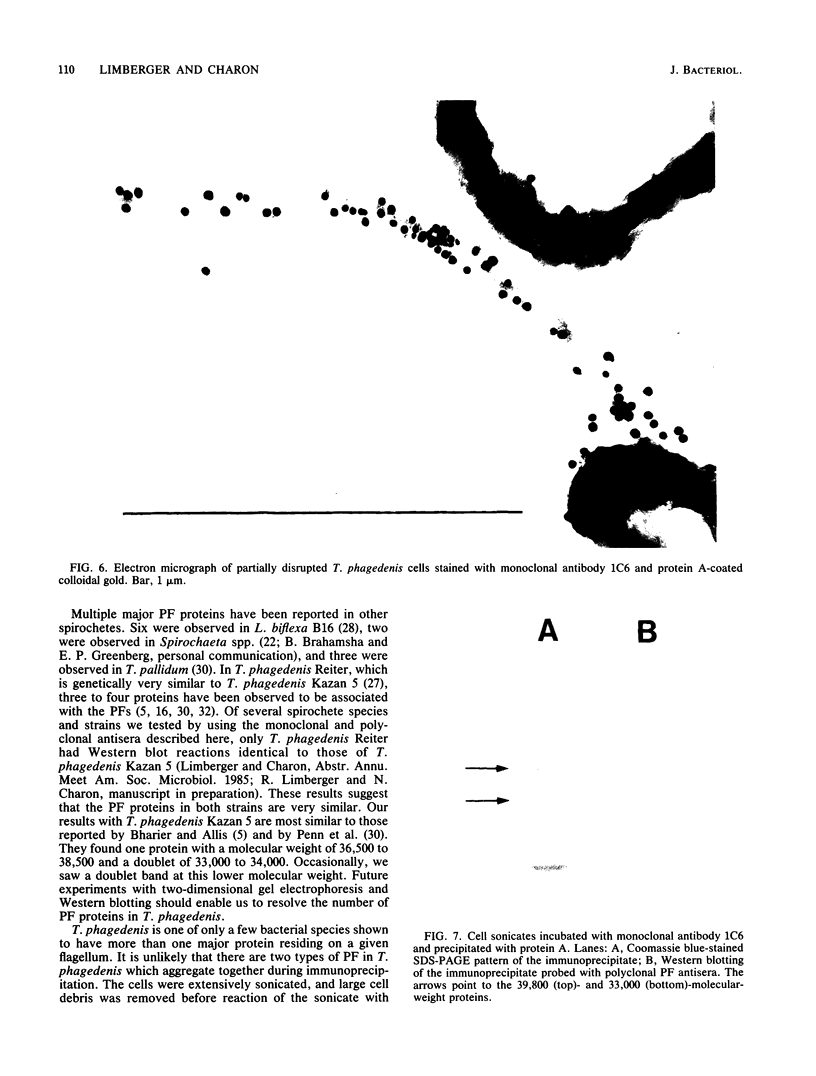
Treponema phagedenis is an anaerobic, motile spirochete with several periplasmic flagella (PFs) at each cell end. This study provides the first genetic evidence that multiple protein species are associated with the PFs. In addition, these proteins were found to reside together on a given PF. Nonmotile mutants which lacked the PFs were isolated, and spontaneous revertants to motility regained the PFs. These results suggest that the PFs are involved in the motility of T. phagedenis. Isolated PFs had two major protein bands with molecular weights of 33,000 and 39,800, as revealed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Western blots with monoclonal and polyclonal antibodies indicated that both proteins were absent in the PF mutants but present in the revertants. Immunoelectron microscopy revealed that the 39,800-molecular-weight protein was distributed along the entire PF. Immunoprecipitation analysis suggested that the 39,800- and 33,000-molecular-weight proteins were closely associated in situ.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour A. G., Tessier S. L., Hayes S. F. Variation in a major surface protein of Lyme disease spirochetes. Infect Immun. 1984 Jul;45(1):94–100. doi: 10.1128/iai.45.1.94-100.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Todd W. J. Lyme disease spirochetes and ixodid tick spirochetes share a common surface antigenic determinant defined by a monoclonal antibody. Infect Immun. 1983 Aug;41(2):795–804. doi: 10.1128/iai.41.2.795-804.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg H. C. How spirochetes may swim. J Theor Biol. 1976 Feb;56(2):269–273. doi: 10.1016/s0022-5193(76)80074-4. [DOI] [PubMed] [Google Scholar]
- Bharier M. A., Rittenberg S. C. Chemistry of axial filaments of Treponema zuezerae. J Bacteriol. 1971 Jan;105(1):422–429. doi: 10.1128/jb.105.1.422-429.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharier M., Allis D. Purification and characterization of axial filaments from Treponema phagedenis biotype reiterii (the Reiter treponeme). J Bacteriol. 1974 Dec;120(3):1434–1442. doi: 10.1128/jb.120.3.1434-1442.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromley D. B., Charon N. W. Axial filament involvement in the motility of Leptospira interrogans. J Bacteriol. 1979 Mar;137(3):1406–1412. doi: 10.1128/jb.137.3.1406-1412.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Schachter J. Antigenic analysis of the major outer membrane protein of Chlamydia spp. Infect Immun. 1982 Mar;35(3):1024–1031. doi: 10.1128/iai.35.3.1024-1031.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canale-Parola E. Motility and chemotaxis of spirochetes. Annu Rev Microbiol. 1978;32:69–99. doi: 10.1146/annurev.mi.32.100178.000441. [DOI] [PubMed] [Google Scholar]
- Caterson B., Christner J. E., Baker J. R. Identification of a monoclonal antibody that specifically recognizes corneal and skeletal keratan sulfate. Monoclonal antibodies to cartilage proteoglycan. J Biol Chem. 1983 Jul 25;258(14):8848–8854. [PubMed] [Google Scholar]
- DePamphilis M. L., Adler J. Purification of intact flagella from Escherichia coli and Bacillus subtilis. J Bacteriol. 1971 Jan;105(1):376–383. doi: 10.1128/jb.105.1.376-383.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eipert S. R., Black S. H. Characterization of the cytoplasmic fibrils of Treponema refringens (Nichols). Arch Microbiol. 1979 Mar 12;120(3):205–214. doi: 10.1007/BF00423067. [DOI] [PubMed] [Google Scholar]
- Emerson S. U., Simon M. I. Variation in the primary structure of Bacillus subtilis flagellins. J Bacteriol. 1971 Jun;106(3):949–954. doi: 10.1128/jb.106.3.949-954.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy P. H., Jr, Fredericks W. R., Nell E. E. Isolation and antigenic characteristics of axial filaments from the Reiter Treponeme. Infect Immun. 1975 Feb;11(2):380–386. doi: 10.1128/iai.11.2.380-386.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C. Anatomy and chemistry of spirochetes. Microbiol Rev. 1978 Mar;42(1):114–160. doi: 10.1128/mr.42.1.114-160.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougen K. H., Birch-Andersen A. Electron microscopy of endoflagella and microtubules in Treponema reiter. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(1):37–50. doi: 10.1111/j.1699-0463.1971.tb00031.x. [DOI] [PubMed] [Google Scholar]
- Hougen K. H. The ultrastructure of cultivable treponemes. 1. Treponema phagedenis, Treponema vincentii and Treponema refringens. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Jun;82(3):329–344. [PubMed] [Google Scholar]
- Hovind-Hougen K. Determination by means of electron microscopy of morphological criteria of value for classification of some spirochetes, in particular treponemes. Acta Pathol Microbiol Scand Suppl. 1976;(255):1–41. [PubMed] [Google Scholar]
- Johnson R. C., Wachter M. S., Ritzi D. M. Treponeme outer cell envelope: solubilization and reaggregation. Infect Immun. 1973 Feb;7(2):249–258. doi: 10.1128/iai.7.2.249-258.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph R., Canale-Parola E. Axial fibrils of anaerobic spirochetes: ultrastructure and chemical characteristics. Arch Mikrobiol. 1972;81(2):146–168. doi: 10.1007/BF00412325. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lagenaur C., Agabian N. Physical characterization of Caulobacter crescentus flagella. J Bacteriol. 1976 Oct;128(1):435–444. doi: 10.1128/jb.128.1.435-444.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Maza O., Fehniger T. E., Ashman R. F. Antibody-secreting cell precursor frequencies among the sheep-erythrocyte-binding cells after immunization. Scand J Immunol. 1983 Apr;17(4):345–354. doi: 10.1111/j.1365-3083.1983.tb00799.x. [DOI] [PubMed] [Google Scholar]
- Miao R., Fieldsteel A. H. Genetics of Treponema: relationship between Treponema pallidum and five cultivable treponemes. J Bacteriol. 1978 Jan;133(1):101–107. doi: 10.1128/jb.133.1.101-107.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauman R. K., Holt S. C., Cox C. D. Purification, ultrastructure, and composition of axial filaments from Leptospira. J Bacteriol. 1969 Apr;98(1):264–280. doi: 10.1128/jb.98.1.264-280.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paster B. J., Canale-Parola E. Involvement of periplasmic fibrils in motility of spirochetes. J Bacteriol. 1980 Jan;141(1):359–364. doi: 10.1128/jb.141.1.359-364.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn C. W., Bailey M. J., Cockayne A. The axial filament antigen of Treponema pallidum. Immunology. 1985 Apr;54(4):635–641. [PMC free article] [PubMed] [Google Scholar]
- Petersen C. S., Pedersen N. S., Axelsen N. H. A simple method for the isolation of flagella from Treponema Reiter. Acta Pathol Microbiol Scand C. 1981 Dec;89(6):379–385. doi: 10.1111/j.1699-0463.1981.tb02716.x. [DOI] [PubMed] [Google Scholar]
- RYTER A., PILLOT J. [Electron microscope study of the external and internal structure of the Reiter treponema]. Ann Inst Pasteur (Paris) 1963 Apr;104:496–501. [PubMed] [Google Scholar]
- Shinoda S., Honda T., Takeda Y., Miwatani T. Antigenic difference between polar montrichous and peritrichous flagella of Vibrio parahaemolyticus. J Bacteriol. 1974 Nov;120(2):923–928. doi: 10.1128/jb.120.2.923-928.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M., Simon M. I. Bacterial flagella. Annu Rev Microbiol. 1977;31:397–419. doi: 10.1146/annurev.mi.31.100177.002145. [DOI] [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J. Sizing of protein A-colloidal gold probes for immunoelectron microscopy. J Cell Biol. 1981 Aug;90(2):533–536. doi: 10.1083/jcb.90.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes J. A., Miller J. N. Ultrastructural studies of treponemes: location of axial filaments and some dimensions of Treponema pallidum (Nichols strain), Treponema denticola, and Treponema reiteri. Infect Immun. 1973 Jan;7(1):100–110. doi: 10.1128/iai.7.1.100-110.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weissborn A., Steinmann H. M., Shapiro L. Characterization of the proteins of the Caulobacter crescentus flagellar filament. Peptide analysis and filament organization. J Biol Chem. 1982 Feb 25;257(4):2066–2074. [PubMed] [Google Scholar]










