Abstract
The GNAS1 gene encodes the α subunit of the G protein Gs, which couples receptor binding by several hormones to activation of adenylate cyclase. Null mutations of GNAS1 cause pseudohypoparathyroidism (PHP) type Ia, in which hormone resistance occurs in association with a characteristic osteodystrophy. The observation that PHP Ia almost always is inherited maternally has led to the suggestion that GNAS1 may be an imprinted gene. Here, we show that, although Gsα expression (directed by the promoter upstream of exon 1) is biallelic, GNAS1 is indeed imprinted in a promoter-specific fashion. We used parthenogenetic lymphocyte DNA to screen by restriction landmark genomic scanning for loci showing differential methylation between paternal and maternal alleles. This screen identified a region that was found to be methylated exclusively on a maternal allele and was located ≈35 kb upstream of GNAS1 exon 1. This region contains three novel exons that are spliced into alternative GNAS1 mRNA species, including one exon that encodes the human homologue of the large G protein XLαs. Transcription of these novel mRNAs is exclusively from the paternal allele in all tissues examined. The differential imprinting of separate protein products of GNAS1 therefore may contribute to the anomalous inheritance of PHP Ia.
Hormone signaling through many cell surface receptors is coupled to cyclic AMP generation, via the heterotrimeric GTP-binding protein Gs, which stimulates adenylate cyclase. The α subunit of Gs (Gsα) is encoded by the GNAS1 gene, located on chromosome 20q13.11 and originally reported to consist of 13 exons (1). Heterozygous null mutations of GNAS1, with reduced red cell Gsα activity, are found in patients with pseudohypoparathyroidism type Ia (PHP Ia) (2–4). However, analysis of published pedigrees has indicated that PHP Ia almost always is inherited maternally (5), suggesting that GNAS1 might be an imprinted gene. Despite this clinical observation, we have shown previously that GNAS1 is biallelically expressed in a wide range of human fetal tissues (6). Although monoallelic expression of GNAS1 confined to particular cell types or developmental stages has not been excluded rigorously, to date the only molecular evidence in favor of imprinting of the gene is from studies of murine Gnas1. A difference in intensity of in situ hybridization signals in the renal glomerulus was found between mice with paternal or maternal uniparental disomy for the region of chromosome 2 containing Gnas1, suggesting a predominantly paternal origin of glomerular Gnas1 transcripts (7).
Here, in contrast, we show that human GNAS1 is indeed imprinted but that this imprinting is promoter-specific. Novel GNAS1 exons ≈35 kb upstream of the originally described exon 1 lie within a region of allele-specific methylation. Transcripts from this region are alternatively spliced onto exon 2, yielding mRNA species that are derived exclusively from the paternal allele, including one that encodes a protein homologous to the large Gsα-related protein XLαs of the rat (8). The XLαs promoter is active only on a paternal allele, even in the same tissue samples in which transcription from the major promoter (upstream of exon 1) is biallelic.
MATERIALS AND METHODS
Restriction Landmark Genomic Scanning.
The screening method using human parthenogenetic material will be detailed elsewhere. In brief, peripheral blood DNA from patient F.D. (9) or from age-matched normal Scottish females was analyzed by RLGS-M (10) by using the enzyme combination AscI—EcoRV–MboI. The A20 spot, identified by being absent (methylated) in F.D. DNA but present at haploid intensity in all controls, was cloned into pBluescriptII (Stratagene) as described (11).
DNA Sequencing.
Sequencing was performed by using [33P]-ddNTP or dye-terminator cycle sequencing kits (both Amersham). image cDNA clone 359933 was identified by blast searching (http://www.ncbi.nlm.nih.gov/BLAST/) with the A20 genomic clone. It then was obtained from the MRC Human Genome Mapping Project (HGMP) Resource Centre and completely sequenced.
Genomic Cloning.
The RPCI1 human PAC library (from the HGMP Resource Centre) was screened by PCR for the presence of GNAS1 exon 7. Four clones (60o16, 63n2, 110p14, and 309f20) were isolated. One (309f20) was positive on rescreening for the presence of exon A20. Subcloned BamHI and SacI–AscI fragments containing this exon were sequenced.
Methylation Analysis.
A 46,XX parthenogenetic lymphoblastoid cell line was established from patient F.D. and used to prepare genomic DNA for Southern blotting by standard methods (9).
RT-PCR Analysis.
Collection of first trimester fetal material and genotyping for a FokI polymorphism in GNAS1 exon 5 were described (6). For reverse transcription, RNA samples and 500 nM primer dGAGCTCGAGTCGACATCGA(T)17 in water were incubated at 85°C for 5 min and then placed on ice. The reverse transcription components then were added, giving a 30-μl reaction of 50 mM Tris⋅HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 10 mM DTT, 1 mM each dNTP, 100 units of Superscript II (GIBCO/BRL), and 39 units of RNAguard (Pharmacia). This was incubated for 60 min at 42°C, 15 min at 50°C, and 15 min at 95°C. This RT mix (2 μl) was amplified in a 50-μl reaction containing 10 mM Tris⋅HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 300 nM each primer, 200 μM each dNTP, and 25 units/ml recombinant Taq polymerase. Cycling conditions were 5 min hot start 94°C, followed by 40 cycles (94°C, 64°C, 72°C for 45 s, 45 s, 60 s). All reactions included a common downstream exon 6 primer (dCCTTGGCATGCTCATAGAATTC). The upstream primer was located in exon 2 (dACCATTGTGAAGCAGATGAGGAT), exon 1 (dCCATGGGCTGCCTCGGGAACA), or the XLαs exon (dGGATGCCTCCGCTGGTTTCAG). PCR product (15 μl) was digested with 1 unit of FokI (New England Biolabs) for 2 h at 37°C, followed by heat inactivation at 65°C for 15 min, ethanol precipitation, and analysis on 2% agarose gels.
RESULTS
Identification of GNAS1 in a Screen for Imprinted Genes.
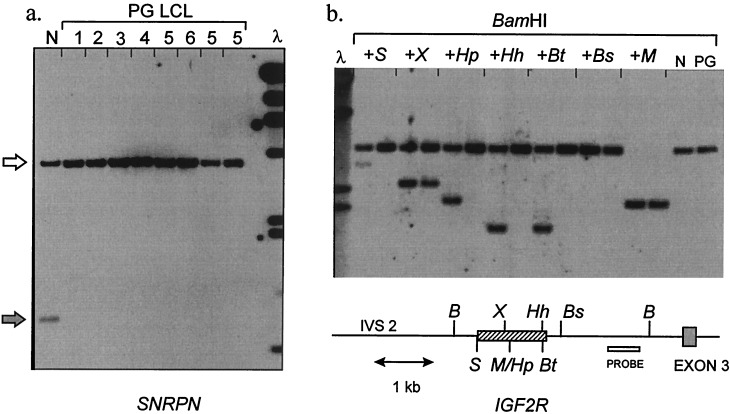
That GNAS1, despite its biallelic transcription (6), might indeed be imprinted was indicated to us by its identification in a screen for unknown imprinted genes. We devised an approach to screening for differentially methylated loci, by using DNA from an individual (F.D.) whose peripheral blood leucocytes are parthenogenetic and isodisomic (9). By Southern blotting for known imprinted loci (SNRPN, chromosome 15q11-q13; IGF2R, chromosome 6q26), we first confirmed our prediction that F.D. lymphoblastoid cell DNA should lack paternal-specific methylation patterns (Fig. 1). This suggested that screening for methylation differences between F.D. and normal DNA then might be used to identify unknown imprinted human genes. Unlike other human parthenogenetic sources (ovarian teratomas) in which uniparental methylation patterns are not faithfully retained (12, 13), use of parthenogenetic leukocyte or lymphoblastoid cell DNA was expected to allow precise matching for cell type and age with normal DNA samples (14).
Figure 1.
Uniparental methylation pattern at imprinted loci in human parthenogenetic LCL. (a) Methylation of SNRPN (chromosome 15q11-q13). The probe used for Southern blotting is KB17, a standard diagnostic reagent for Prader–Willi syndrome. DNAs are digested with XbaI + NotI. The paternal allele (gray arrow) is unmethylated, yielding a 0.9-kb fragment from normal DNA (N). 1–6, six subcultured lines of parthenogenetic lymphoblastoid cells (LCL) established from patient F.D. Only the methylated 4.2-kb maternal band (white arrow) is present. λ, λHindIII marker; 23, 9.4, 6.6, 4.4, 2.3, 2.0, 0.56 kb. (b) Methylation of IGF2R. The probe (open box) is fragment Bx of clone pE3UP (25), which detects a 3.2-kb BamHI fragment. Pairs of lanes contain normal (N) or parthenogenetic (PG) LCL DNA digested with BamHI, alone or plus a second enzyme as indicated: B, BamHI; S, SacII; X, XhoI; M/Hp, MspI/HpaII; Hh, HhaI; Bt, BstUI; Bs, BssHII. For each enzyme only the site nearest the probe is shown. The cross-hatched box indicates the region of allele-specific methylation; the HpaII, HhaI, and BstUI sites are methylated only on a maternal allele; the SacII site in addition is methylated incompletely on a paternal allele; the XhoI is methylated incompletely on a maternal allele and unmethylated on a paternal allele. These results concur with previous data (25) and indicate faithful allele-specific methylation across this region in the PG LCL DNA. λ, λHindIII marker; 6.6, 4.4, 2.3, 2.0 kb.
By using restriction landmark genomic scanning (15–17), we compared DNA from F.D., from six age-matched, normal Scottish females, and from a complete hydatidiform mole. A differentially methylated spot (A20), absent from F.D. but present at haploid intensity in all control females, was cloned and sequenced. Database searching showed that this 232-nt MboI–AscI fragment (corresponding to nucleotides 1,661–1,892 of deposition AJ224868) contained a single 91-nt exon, as indicated by the fact that this 91-nt region also forms part of an image cDNA clone, 359933 (827-k06).
When completely sequenced, cDNA clone 359933 unexpectedly proved to be derived from the GNAS1 gene; it consists of: (i) a 376-nt upstream region, which further database searching showed to be homologous to part of the 5′ portion of the mRNA encoding the rat Gsα-related protein XLαs (8); (ii) the 91-nt A20 exon; (iii) another previously unidentified 67-nt exon, A21; (iv) GNAS1 exons 2 and 3; and (v) a variant terminal exon (3N) and polyadenylation site, located in intron 3 and homologous to the novel GsαN1 terminal exon described in the rat (18).
Structure and Methylation of the Imprinted Region of GNAS1.
In the rat, XLαs is a 78-kDa protein encoded by an mRNA in which variant 5′ sequences are spliced onto Gnas1 exon 2; its expression appears specific to certain endocrine secretory cells and neurons (8). In human cDNA clone 359933, the XLαs-like sequences are separated from GNAS1 exon 2 by exons A20 and A21. As discussed below, this cDNA is unlikely to encode a functional protein, both because it is truncated at its 3′ end and because the A20-A21 region shifts the frame between XLαs and exon 2 and introduces a stop codon in frame with the exon 2–3 ORF. Nonetheless, the presence of both XLαs-homologous and exon 2–3 sequences indicated that cDNA 359933 represented a genuine GNAS1 transcript. To examine the underlying genomic structure, PAC clones containing GNAS1 were isolated, one of which (309f20) also contained the A20 exon.
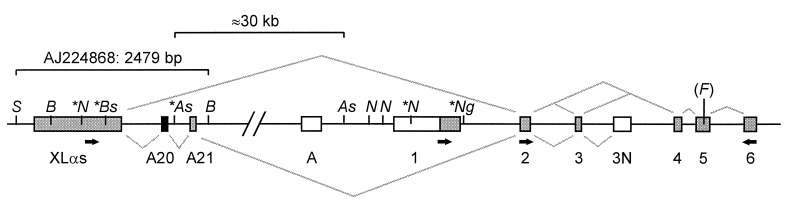
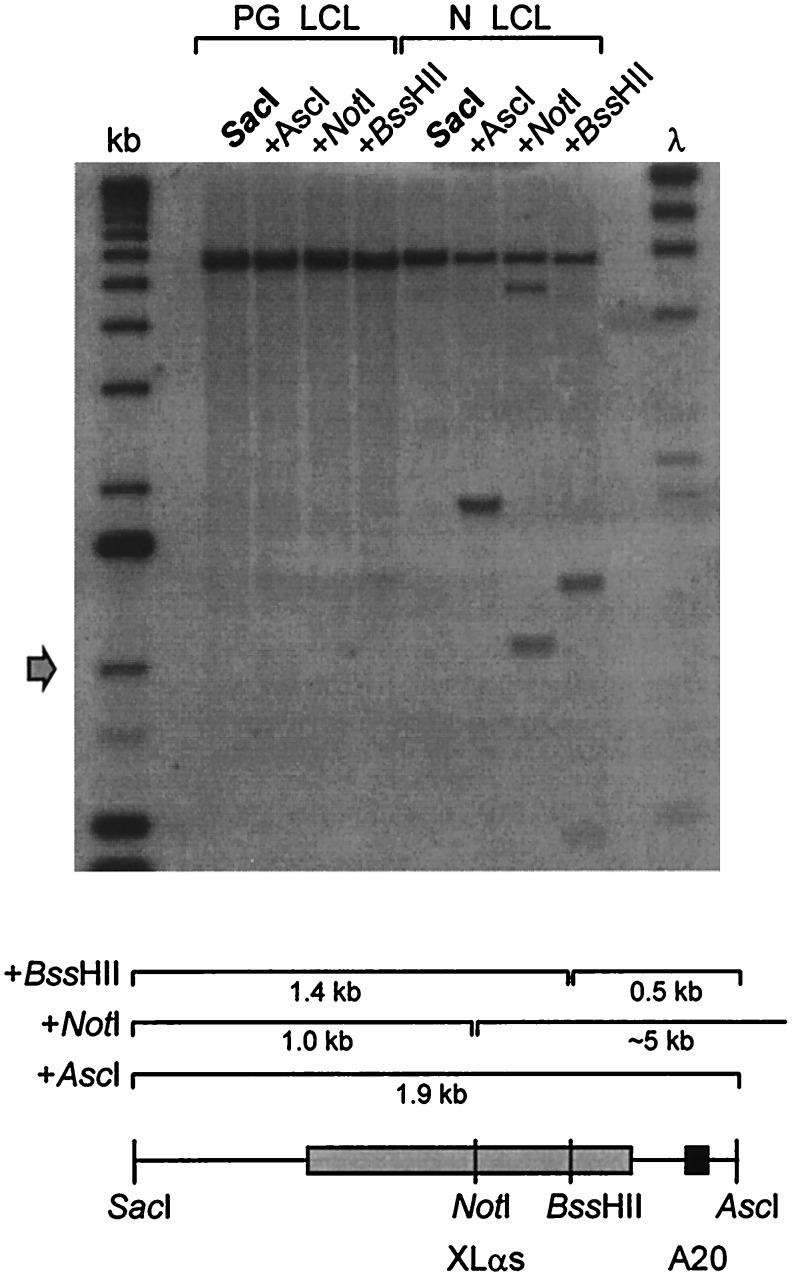
A 2.5-kb region containing XLαs, A20, and A21 exons was sequenced. The XLαs-homologous region is encoded in a single large exon lying ≈35 kb upstream of exon 1 (Fig. 2). A 1.9-kb SacI–AscI fragment was used as probe in Southern blotting of lymphoblastoid cell DNA to assess the methylation status of the NotI, BssHII, and AscI sites in this region (Figs. 2 and 3). Normal DNA yielded bands indicating the presence of both methylated and unmethylated alleles at all three restriction sites. Parthenogenetic DNA, in contrast, was completely methylated at all three sites. This confirms that the XLαs-A20 region of GNAS1 is differentially methylated, the paternal allele being unmethylated and the maternal methylated. In contrast to the differential methylation of the XLα region, Southern blotting showed that NotI and NgoMI sites in the vicinity of exon 1 (Fig. 2) were unmethylated on both maternal and paternal alleles (data not shown).
Figure 2.
Organization of the XLαs region of GNAS1. The map is not to scale, and not all restriction sites are shown, with the exception of AscI and NotI. Grey lines above the exons represent the splicing patterns observed in the majority of XLαs-containing RT-PCR products and below the line the splicing pattern seen in the 359933 cDNA clone. Italic letters indicate restriction sites: S, SacI; B, BamHI; N, NotI; As, AscI; Ng, NgoMI; Bs, BssHII; (F), FokI (polymorphic site). Asterisks indicate those sites whose methylation status was assessed by Southern blotting (see text). The distance between the two AscI sites is ≈30 kb. The 2.5-kb genomic region that was sequenced is indicated. The A20 exon is black; other exons are gray or white for untranslated regions. The exon labeled A has been described (26). Small black arrows indicate approximate positions of primers used for RT-PCR.
Figure 3.
Differential methylation in the XLαs region of GNAS1. The probe used was a 1.9-kb SacI–AscI genomic fragment containing both the XLαs and A20 exons (shaded boxes). Genomic DNA from parthenogenetic (left) or normal (right) lymphoblastoid cell DNA was digested with SacI, alone or in combination with AscI, NotI, or BssHII. SacI generates a fragment of ≈6 kb; the expected fragment sizes derived from additional cleavage at the methylation sensitive sites are indicated on the diagram. λ, λHindIII marker; 23, 9.4, 6.6, 4.4, 2.3, 2.0, 0.56 kb. kb, 1-kb ladder (GIBCO/BRL); upwards from arrow 1.0, 1.6, 2.0, 3.1, 4.1, 5.1, 6.1, 7.1 kb.
Monoallelic Expression of XLαs-Containing Transcripts.
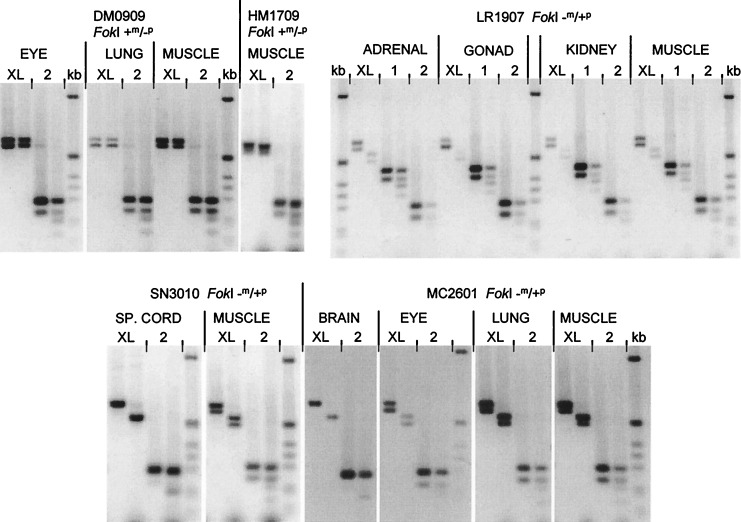
To determine whether the differential methylation of GNAS1 reflects monoallelic expression, RT-PCR analysis of fetal RNA samples was performed. Previously, we had used RNA from first trimester fetuses, informative for a FokI polymorphism in exon 5, to show that GNAS1 mRNA (amplified by using primers in exons 4 and 5) was derived equally from both alleles in all tissues surveyed (6). RNA samples from seven heterozygous fetuses now were reanalyzed by RT-PCR between exons 2 and 6, followed by FokI digestion. This assay, which examines total expression of all of the major GNAS1 species, regardless of which exon is spliced in front of exon 2, confirmed biallelic expression in all samples (Fig. 4, all pairs of lanes labeled 2). When, however, RT-PCR was performed by using an upstream primer in the XLαs exon rather than exon 2, FokI digestion revealed that all of the XLαs-containing transcripts were derived from the paternal allele (Fig. 4, pairs of lanes labeled XL). Identical results were obtained for several fetuses, both those with a +m/−p genotype (Fig. 4, fetuses DM0909, HM1709) in which the XL RT-PCR products were completely FokI-resistant and those with a −m/+p genotype (Fig. 4, fetuses LR1907, SN3010, MC2601) in which the XL RT-PCR products were cleaved to completion by FokI. The XLαs mRNA was derived from the paternal allele in all tissues and in all seven fetuses examined. Exon 1-specific products, in contrast, were derived from both alleles (Fig. 4, LR1907, lanes labeled 1). Thus, the biallelic expression seen using the exon 2 primer does not simply reflect a mixture of transcripts derived from one paternally active (i.e., XLαs exon) and one maternally active (i.e., exon 1) promoter. Some of the ethidium-stained cleavage products of exon 2-exon 6 products in Fig. 4 appear faint. However, careful quantitation by 32P-labeling and phosphorimaging or fluorescent primer labeling has shown that, within the limits of experimental error, the “pooled” GNAS1 transcripts represented by these RT-PCR products are derived equally from maternal and paternal alleles (6).
Figure 4.
Exclusively paternal expression of XLαs-containing GNAS1 transcripts. All experiments used a downstream PCR primer in exon 6; the location of the upstream primer is indicated (either in the XLαs exon, exon 1 or exon 2) above each pair of lanes. Each pair of lanes contains undigested (Left) or FokI-digested (Right) RT-PCR product. The two bands seen in most undigested samples result from alternative splicing of exon 3. In fetuses DM0909 and HM1709, the paternal allele is lacking a FokI site (genotype +m/−p). The XLαs-derived RT-PCR products (XL) are completely FokI-resistant. The total GNAS1 transcripts, in contrast (2), are partially cleaved by FokI, indicating their biallelic origin. In fetus LR1907, the genotype is −m/+p. The XLαs-derived products are digested to completion by FokI, again demonstrating their exclusively paternal origin. Also, in this panel, exon 1-specific transcription has been analyzed separately, showing biallelic expression from the promoter upstream of exon 1. Fetuses SN3010 and MC2601 are again FokI −m/+p. RT-PCR products are digested to completion if XLαs-derived but only partly when the exon 2 primer is used. kb, 1-kb ladder: 1,018, 517/506, 396, 344, 298, 220, 201, 154, 134 bp.
The XLαs-exon 6 RT-PCR products were considerably shorter than the 783 bp predicted from the sequence of the 359933 cDNA clone. Sequencing of these transcripts confirmed that, in them, as in the rat XLαs mRNA, the XLαs exon is spliced directly onto exon 2, resulting in a mRNA with a continuous ORF homologous to that of the rat mRNA. RT-PCR products containing A20 or A21 were not detected in these experiments, suggesting that most XLαs-containing transcripts do not contain the A20/A21 exons. Because A20/A21-containing transcripts also do not have a continuous ORF, they may be aberrant or functionally unimportant splice forms. Additional RT-PCR experiments using a primer specific for A20 did nonetheless show that A20-containing transcripts also are derived exclusively from the paternal allele (not shown).
In almost all RT-PCR experiments, a doublet band consisting of two products differing in size by 45 bp was obtained (Fig. 4). These derive from alternatively spliced mRNAs (1) including or excluding exon 3. Both XLαs- and Gsα-encoding transcripts show this alternative splicing. In some individual RNA samples, however (including all three spinal cords examined), only the larger exon 3-containing mRNA was detected (Fig. 4, SN3010 spinal cord, MC2601 brain).
Sequence Conservation and Repetitive Elements in the XLαs Region.
Attempts to map the 5′ end of the XLαs-containing transcripts by RACE were unsuccessful, probably because of the GC-richness of this region. However, RT-PCR products diagnostic of XLαs-exon 2 spliced mRNA could be generated by using upstream primers as much as 1.1 kb from the 3′ end of the XLαs exon. (nucleotides 375–1,482 of accession number AJ224868; data not shown). These experiments indicated that the XLαs exon is >1.1 kb in size. It may extend even further 5′ because the reading frame remains open beyond the start of our sequence (1,482 nt upstream of the 3′ end of the XLαs exon). In Fig. 5, the translated human ORF (starting at nucleotide 318 of AJ224868) is aligned with the peptide sequence predicted by a full length rat cDNA (8). There are well conserved regions at the N- and C-terminal extremes of the XLαs segments. The central region aligns much less well, but there are nonetheless qualitative similarities between the two species in this region, which in both human and rat contains a repetitive domain that interrupts the conserved unique regions. (There is also an additional insertion of ≈300 nt in the rat sequence.) In this repetitive region, the rat XLαs protein has a tetrapeptide repeat based on EPAA (single letter amino acid code). The predicted human protein has a tripeptide repeat motif, which is again rich in proline, alanine, and an acidic amino acid (aspartate rather than glutamate). There is also a higher order repeat of 9 or 12 amino acids (Fig. 6). The overall ratio of D+E:P:A in this region (20:22:42) is virtually identical to that in the rat EPAA repeat region.
Figure 5.
Comparison between human (Upper) and rat (Lower) XLαs peptide sequences. Extensive homology is confined to the three shaded regions, whereas, as discussed in the text, the central repetitive region aligns poorly between species. Translation of the human exon has been started at position 318 relative to the SacI site (AJ224868). However, the true translation initiation site has not been defined experimentally either for human or rat XLαs (8).
Figure 6.
Internal repeat motifs in the human XLαs exon. Almost the whole of the central poorly conserved region is included in this repeat, which begins at residue 74 and ends at residue 225, immediately before the second highly conserved region (shaded in Fig. 5).
Although the function of the repeated elements of XLαs is undefined, there are interesting parallels with the structure of the p57KIP2 protein, encoded by another imprinted gene, CDKN1C (19, 20). Murine p57KIP2 contains a proline-rich domain that overlaps with an acidic domain containing multiple tetrapeptide repeats based on the motif EPVE. In human p57KIP2, these domains are replaced by a simpler repeat, based on PAPA (21, 22). As in XLαs, the functions of these acidic or proline/alanine rich repeats and the reason for their poor conservation between human and rodent, are unclear. However, because the XLαs region is GC-rich and repetitive, like several other regions of differential methylation in imprinted genes, it is possible that selection to maintain these features operates at the DNA rather than the protein level (23).
DISCUSSION
Based on studies of multi-generation families with PHP/PPHP, only maternal inheritance appears to result in the full blown PHP syndrome, with hormone resistance (5). This observation is supported strongly by the pedigree data presented in a more recent study of a large number of families (even though its authors did not favor the imprinting hypothesis) (24). That maternal inheritance of GNAS1 mutations may be required for development of PHP Ia suggests the existence of maternally expressed GNAS1 transcripts. It is therefore unexpected that we have now demonstrated specific paternal expression from a GNAS1 promoter. It remains possible that other, maternally derived GNAS1 transcripts with distinct functions could exist or even that there is maternal-only transcription from exon 1 at discrete developmental stages or in subpopulations of hormone-responsive cells. Despite this, the possibility that XLαs is involved in the pathogenesis of PHP Ia also merits further investigation. However, for this to be consistent with the maternal inheritance pattern of PHP, one would have to explain how a paternally inherited mutation, resulting in both reduced Gsα and loss of XLαs, could have a less severe effect than a maternal one, resulting in reduced Gsα activity alone. Additional studies of the biochemical and physiological function of XLαs will be needed before definite conclusions can be drawn.
In the mouse, in situ hybridization has suggested preferential expression of the paternal Gnas1 allele but confined to the renal glomerulus (7). We, in contrast, have shown here that promoter-specific imprinting of GNAS1 in human fetal tissues is not tissue-specific. It is possible that a re-evaluation of murine Gnas1 imprinting in such a way as to distinguish different transcript species would show evolutionary conservation of the paternal origin of XLαs-containing transcripts. If so, it also may be possible to test hypotheses about the relationship between XLαs and PHP Ia by introducing germ-line mutations into the murine XLαs exon.
Acknowledgments
This work was supported by the Ludovici Bequest to the University of Edinburgh; by Wellcome Trust Project Grant 053701 to D.T.B.; by Core Research for Evolutional Science and Technology (CREST) from the Japan Science and Technology Corporation, Special Coordination Funds and a Research Grant for the Genome Exploration Research Project from the Science and Technology Agency of the Japanese Government, and by a Grant-in-Aid for Scientific Research on Priority Areas and Human Genome Program from the Ministry of Education and Culture, Japan to Y.H. V.M. is supported by a scholarship from CONACYT (Mexican National Council for Science and Technology).
ABBREVIATIONS
- PHP
pseudohypoparathyroidism
- RLGS-M
restriction landmark genomic scanning for methylation
Footnotes
References
- 1.Kozasa T, Itoh H, Tsukamoto T, Kaziro Y. Proc Natl Acad Sci USA. 1988;85:2081–2085. doi: 10.1073/pnas.85.7.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine M A, Ahn T G, Klupt S F, Kaufman K D, Smallwood P M, Bourne H R, Sullivan K A, Van Dop C. Proc Natl Acad Sci USA. 1988;85:617–621. doi: 10.1073/pnas.85.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patten J L, Johns D R, Valle D, Eil C, Grupposo P A, Steele G, Smallwood P M, Levine M A. N Engl J Med. 1990;322:1412–1419. doi: 10.1056/NEJM199005173222002. [DOI] [PubMed] [Google Scholar]
- 4.Weinstein L S, Gejman P V, Collins R M, Gershon E S, Spiegel A M. Proc Natl Acad Sci USA. 1990;87:8287–8290. doi: 10.1073/pnas.87.21.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies S J, Hughes H E. J Med Genet. 1993;30:101–103. doi: 10.1136/jmg.30.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell R, Gosden C M, Bonthron D T. J Med Genet. 1994;31:607–614. doi: 10.1136/jmg.31.8.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williamson C M, Schofield J, Dutton E R, Seymour A, Beechey C V, Edwards Y H, Peters J. Genomics. 1996;36:280–287. doi: 10.1006/geno.1996.0463. [DOI] [PubMed] [Google Scholar]
- 8.Kehlenbach R H, Matthey J, Huttner W B. Nature (London) 1994;372:804–809. doi: 10.1038/372804a0. [DOI] [PubMed] [Google Scholar]
- 9.Strain L, Warner J P, Johnston T, Bonthron D T. Nat Genet. 1995;11:164–169. doi: 10.1038/ng1095-164. [DOI] [PubMed] [Google Scholar]
- 10.Hatada I, Hayashizaki Y, Hirotsune S, Komatsubara H, Mukai T. Proc Natl Acad Sci USA. 1991;88:9523–9527. doi: 10.1073/pnas.88.21.9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okazaki Y, Hirose K, Hirotsune S, Okuizumi H, Sasaki N, Ohsumi T, Yoshiki A, Kusakabe M, Muramatsu M, Kawai J, et al. Proc Natl Acad Sci USA. 1995;92:5610–5614. doi: 10.1073/pnas.92.12.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glenn C C, Saitoh S, Jong M T C, Filbrandt M M, Surti U, Driscoll D J, Nicholls R D. Am J Hum Genet. 1996;58:335–346. [PMC free article] [PubMed] [Google Scholar]
- 13.Mutter G L, Stewart C L, Chaponot M L, Pomponio R J. Am J Hum Genet. 1993;53:1096–1102. [PMC free article] [PubMed] [Google Scholar]
- 14.Asakawa J, Kuick R, Neel J V, Kodaira M, Satoh C, Hanash S M. Electrophoresis. 1995;16:241–252. doi: 10.1002/elps.1150160140. [DOI] [PubMed] [Google Scholar]
- 15.Shibata H, Hirotsune S, Okazaki Y, Komatsubara H, Muramatsu M, Takagi N, Ueda T, Shiroishi T, Moriwaki K, Katsuki M, et al. Mammal Genome. 1994;5:797–800. doi: 10.1007/BF00292016. [DOI] [PubMed] [Google Scholar]
- 16.Plass C, Shibata H, Kalcheva I, Mullins L, Kotelevtseva N, Mullins J, Kato R, Sasaki H, Hirotsune S, Okazaki Y, et al. Nat Genet. 1996;14:106–109. doi: 10.1038/ng0996-106. [DOI] [PubMed] [Google Scholar]
- 17.Hayashizaki Y, Shibata Y, Hirotsune S, Sugino H, Okazaki Y, Sasaki N, Hirose K, Imoto H, Okuizumi H, Muramatsu M, et al. Nat Genet. 1994;6:33–40. doi: 10.1038/ng0194-33. [DOI] [PubMed] [Google Scholar]
- 18.Crawford J A, Mutchler K J, Sullivan B E, Lanigan T M, Clark M S, Russo A F. J Biol Chem. 1993;268:9879–9885. [PubMed] [Google Scholar]
- 19.Hatada I, Mukai T. Nat Genet. 1995;11:204–206. doi: 10.1038/ng1095-204. [DOI] [PubMed] [Google Scholar]
- 20.Hatada I, Ohashi H, Fukushima Y, Kaneko Y, Inoue M, Komoto Y, Okada A, Ohishi S, Nabetani A, Morisaki H, et al. Nat Genet. 1996;14:171–173. doi: 10.1038/ng1096-171. [DOI] [PubMed] [Google Scholar]
- 21.Matsuoka S, Edwards M C, Bai C, Parker S, Zhang P, Baldini A, Harper J W, Elledge S J. Genes Dev. 1995;9:650–662. doi: 10.1101/gad.9.6.650. [DOI] [PubMed] [Google Scholar]
- 22.Lee M-H, Reynisdóttir I, Massagué J. Genes Dev. 1995;9:639–649. doi: 10.1101/gad.9.6.639. [DOI] [PubMed] [Google Scholar]
- 23.Neumann B, Kubicka P, Barlow D P. Nat Genet. 1995;9:12–13. doi: 10.1038/ng0195-12. [DOI] [PubMed] [Google Scholar]
- 24.Marguet C, Mallet E, Basuyau J P, Martin D, Leroy M, Brunelle P. Horm Res. 1997;48:120–130. doi: 10.1159/000185501. [DOI] [PubMed] [Google Scholar]
- 25.Smrzka O W, Faé I, Stöger R, Kurzbauer R, Fischer G F, Henn T, Weith A, Barlow D P. Hum Mol Genet. 1995;4:1945–1952. doi: 10.1093/hmg/4.10.1945. [DOI] [PubMed] [Google Scholar]
- 26.Swaroop A, Agarwal N, Gruen J R, Bick D, Weissman S M. Nucleic Acids Res. 1991;19:4725–4729. doi: 10.1093/nar/19.17.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]