Abstract
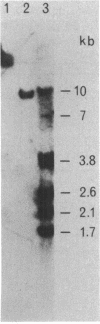
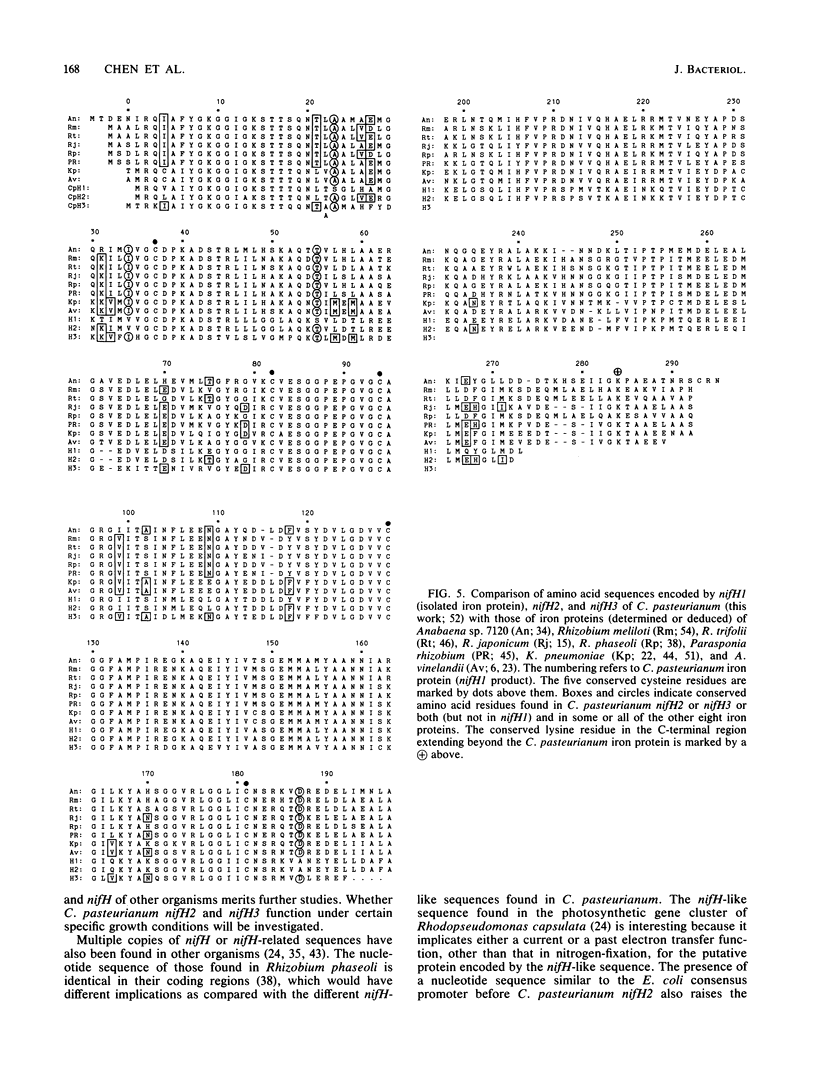
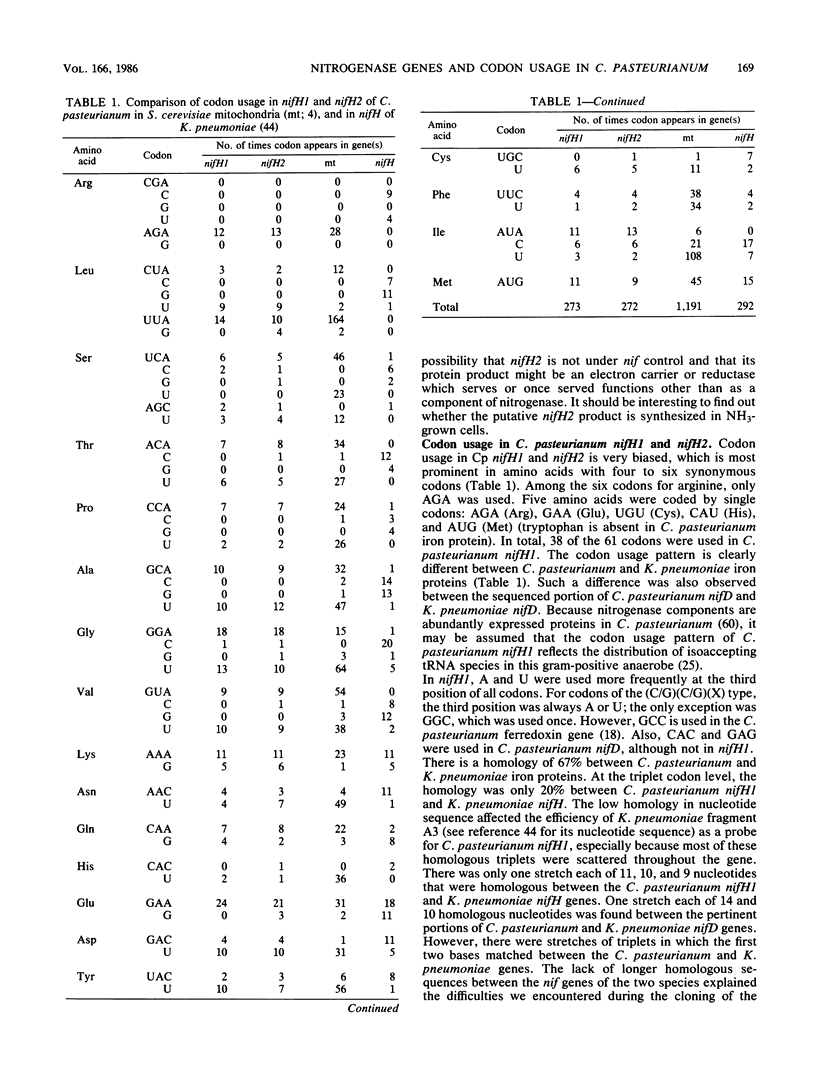
The structural gene (nifH1) encoding the nitrogenase iron protein of Clostridium pasteurianum has been cloned and sequenced. It is located on a 4-kilobase EcoRI fragment (cloned into pBR325) that also contains a portion of nifD and another nifH-like sequence (nifH2). C. pasteurianum nifH1 encodes a polypeptide (273 amino acids) identical to that of the isolated iron protein, indicating that the smaller size of the C. pasteurianum iron protein does not result from posttranslational processing. The 5' flanking region of nifH1 or nifH2 does not contain the nif promoter sequences found in several gram-negative bacteria. Instead, a sequence resembling the Escherichia coli consensus promoter (TTGACA-N17-TATAAT) is present before C. pasteurianum nifH2, and a TATAAT sequence is present before C pasteurianum nifH1. Codon usage in nifH1, nifH2, and nifD (partial) is very biased. A preference for A or U in the third position of the codons is seen. nifH2 could encode a protein of 272 amino acid residues, which differs from the iron protein (nifH1 product) in 23 amino acid residues (8%). Another nifH-like sequence (nifH3) is located on a nonadjacent EcoRI fragment and has been partially sequenced. C. pasteurianum nifH2 and nifH3 may encode proteins having several amino acids that are conserved in other proteins but not in C. pasteurianum iron protein, suggesting a possible role for the multiple nifH-like sequences of C. pasteurianum in the evolution of nifH. Among the nine sequenced iron proteins, only the C. pasteurianum protein lacks a conserved lysine residue which is near the extended C terminus of the other iron proteins. The absence of this positive charge in the C. pasteurianum iron protein might affect the cross-reactivity of the protein in heterologous systems.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonitz S. G., Berlani R., Coruzzi G., Li M., Macino G., Nobrega F. G., Nobrega M. P., Thalenfeld B. E., Tzagoloff A. Codon recognition rules in yeast mitochondria. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3167–3170. doi: 10.1073/pnas.77.6.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigle K. E., Newton W. E., Dean D. R. Complete nucleotide sequence of the Azotobacter vinelandii nitrogenase structural gene cluster. Gene. 1985;37(1-3):37–44. doi: 10.1016/0378-1119(85)90255-0. [DOI] [PubMed] [Google Scholar]
- Béguin P., Cornet P., Aubert J. P. Sequence of a cellulase gene of the thermophilic bacterium Clostridium thermocellum. J Bacteriol. 1985 Apr;162(1):102–105. doi: 10.1128/jb.162.1.102-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon F. C., Riedel G. E., Ausubel F. M. Overlapping sequences of Klebsiella pneumoniae nifDNA cloned and characterized. Mol Gen Genet. 1979 Jul 2;174(1):59–66. doi: 10.1007/BF00433306. [DOI] [PubMed] [Google Scholar]
- Chen J. S., Multani J. S., Mortenson L. E. Structural investigation of nitrogenase components from Clostridium pasteurianum and comparison with similar components of other organisms. Biochim Biophys Acta. 1973 May 17;310(1):51–59. doi: 10.1016/0005-2795(73)90007-x. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Dixon R. A. The genetic complexity of nitrogen fixation. The ninth Fleming lecture. J Gen Microbiol. 1984 Nov;130(11):2745–2755. doi: 10.1099/00221287-130-11-2745. [DOI] [PubMed] [Google Scholar]
- Emerich D. W., Burris R. H. Complementary functioning of the component proteins of nitrogenase from several bacteria. J Bacteriol. 1978 Jun;134(3):936–943. doi: 10.1128/jb.134.3.936-943.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann M., Hennecke H. Rhizobium japonicum nitrogenase Fe protein gene (nifH). J Bacteriol. 1984 Jun;158(3):1005–1011. doi: 10.1128/jb.158.3.1005-1011.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George H. A., Johnson J. L., Moore W. E., Holdeman L. V., Chen J. S. Acetone, Isopropanol, and Butanol Production by Clostridium beijerinckii (syn. Clostridium butylicum) and Clostridium aurantibutyricum. Appl Environ Microbiol. 1983 Mar;45(3):1160–1163. doi: 10.1128/aem.45.3.1160-1163.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves M. C., Mullenbach G. T., Rabinowitz J. C. Cloning and nucleotide sequence determination of the Clostridium pasteurianum ferredoxin gene. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1653–1657. doi: 10.1073/pnas.82.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth J. H., Burris R. H. Inhibition of nitrogenase-catalyzed NH3 formation by H2. Biochemistry. 1983 Oct 25;22(22):5111–5122. doi: 10.1021/bi00291a010. [DOI] [PubMed] [Google Scholar]
- Hase T., Nakano T., Matsubara H., Zumft W. G. Correspondence of the larger subunit of the MoFe-protein in clostridial nitrogenase to the nif D gene products of other N2-fixing organisms. J Biochem. 1981 Jul;90(1):295–298. doi: 10.1093/oxfordjournals.jbchem.a133466. [DOI] [PubMed] [Google Scholar]
- Hase T., Wakabayashi S., Nakano T., Zumft W. G., Matsubara H. Structural homologies between the amino acid sequence of Clostridium pasteurianum MoFe protein and the DNA sequences of nifD and K genes of phylogenetically diverse bacteria. FEBS Lett. 1984 Jan 23;166(1):39–43. doi: 10.1016/0014-5793(84)80040-x. [DOI] [PubMed] [Google Scholar]
- Hausinger R. P., Howard J. B. Comparison of the iron proteins from the nitrogen fixation complexes of Azotobacter vinelandii, Clostridium pasteurianum, and Klebsiella pneumoniae. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3826–3830. doi: 10.1073/pnas.77.7.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausinger R. P., Howard J. B. The amino acid sequence of the nitrogenase iron protein from Azotobacter vinelandii. J Biol Chem. 1982 Mar 10;257(5):2483–2490. [PubMed] [Google Scholar]
- Hearst J. E., Alberti M., Doolittle R. F. A putative nitrogenase reductase gene found in the nucleotide sequences from the photosynthetic gene cluster of R. capsulata. Cell. 1985 Jan;40(1):219–220. doi: 10.1016/0092-8674(85)90325-3. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes: a proposal for a synonymous codon choice that is optimal for the E. coli translational system. J Mol Biol. 1981 Sep 25;151(3):389–409. doi: 10.1016/0022-2836(81)90003-6. [DOI] [PubMed] [Google Scholar]
- Lammers P. J., Haselkorn R. Sequence of the nifD gene coding for the alpha subunit of dinitrogenase from the cyanobacterium Anabaena. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4723–4727. doi: 10.1073/pnas.80.15.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundell D. J., Howard J. B. Isolation and partial characterization of two different subunits from the molybdenum-iron protein of Azotobacter vinelandii nitrogenase. J Biol Chem. 1978 May 25;253(10):3422–3426. [PubMed] [Google Scholar]
- Mazur B. J., Chui C. F. Sequence of the gene coding for the beta-subunit of dinitrogenase from the blue-green alga Anabaena. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6782–6786. doi: 10.1073/pnas.79.22.6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur B. J., Rice D., Haselkorn R. Identification of blue-green algal nitrogen fixation genes by using heterologous DNA hybridization probes. Proc Natl Acad Sci U S A. 1980 Jan;77(1):186–190. doi: 10.1073/pnas.77.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mevarech M., Rice D., Haselkorn R. Nucleotide sequence of a cyanobacterial nifH gene coding for nitrogenase reductase. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6476–6480. doi: 10.1073/pnas.77.11.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pustell J., Kafatos F. C. A convenient and adaptable package of DNA sequence analysis programs for microcomputers. Nucleic Acids Res. 1982 Jan 11;10(1):51–59. doi: 10.1093/nar/10.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinto C., De La Vega H., Flores M., Leemans J., Cevallos M. A., Pardo M. A., Azpiroz R., De Lourdes Girard M., Calva E., Palacios R. Nitrogenase reductase: A functional multigene family in Rhizobium phaseoli. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1170–1174. doi: 10.1073/pnas.82.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts G. P., Brill W. J. Genetics and regulation of nitrogen fixation. Annu Rev Microbiol. 1981;35:207–235. doi: 10.1146/annurev.mi.35.100181.001231. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. Interspecies homology of nitrogenase genes. Proc Natl Acad Sci U S A. 1980 Jan;77(1):191–195. doi: 10.1073/pnas.77.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnik P. A., Haselkorn R. Activation of extra copies of genes coding for nitrogenase in Rhodopseudomonas capsulata. Nature. 1984 Jan 19;307(5948):289–292. doi: 10.1038/307289a0. [DOI] [PubMed] [Google Scholar]
- Scott K. F., Rolfe B. G., Shine J. Biological nitrogen fixation: primary structure of the Klebsiella pneumoniae nifH and nifD genes. J Mol Appl Genet. 1981;1(1):71–81. [PubMed] [Google Scholar]
- Scott K. F., Rolfe B. G., Shine J. Biological nitrogen fixation: primary structure of the Rhizobium trifolii iron protein gene. DNA. 1983;2(2):149–155. doi: 10.1089/dna.1983.2.149. [DOI] [PubMed] [Google Scholar]
- Scott K. F., Rolfe B. G., Shine J. Nitrogenase structural genes are unlinked in the nonlegume symbiont Parasponia rhizobium. DNA. 1983;2(2):141–148. doi: 10.1089/dna.1983.2.141. [DOI] [PubMed] [Google Scholar]
- Shen S. C., Xue Z. T., Kong Q. I., Wu Q. L. An open reading frame upstream from the nifH gene of Klebsiella pneumoniae. Nucleic Acids Res. 1983 Jun 25;11(12):4241–4250. doi: 10.1093/nar/11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. E., Thorneley R. N., Eady R. R., Mortenson L. E. Nitrogenases from Klebsiella pneumoniae and Clostridium pasteurianum. Kinetic investigations of cross-reactions as a probe of the enzyme mechanism. Biochem J. 1976 Aug 1;157(2):439–447. doi: 10.1042/bj1570439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stormo G. D., Schneider T. D., Gold L. M. Characterization of translational initiation sites in E. coli. Nucleic Acids Res. 1982 May 11;10(9):2971–2996. doi: 10.1093/nar/10.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan V., Ausubel F. M. Nucleotide sequence of the gene coding for the nitrogenase iron protein from Klebsiella pneumoniae. J Biol Chem. 1981 Mar 25;256(6):2808–2812. [PubMed] [Google Scholar]
- Tanaka M., Haniu M., Yasunobu K. T. The amino acid sequence of Clostridium pasteurianum iron protein, a component of nitrogenase. III. The NH2-terminal and COOH-terminal sequences, tryptic peptides of large cyanogen bromide peptides, and the complete sequence. J Biol Chem. 1977 Oct 25;252(20):7093–7100. [PubMed] [Google Scholar]
- Tsai L. B., Mortenson L. E. Interaction of the nitrogenase components of Anabaena cylindrica with those of Clostridium pasteurianum. Biochem Biophys Res Commun. 1978 Mar 30;81(2):280–287. doi: 10.1016/0006-291x(78)91530-9. [DOI] [PubMed] [Google Scholar]
- Török I., Kondorosi A. Nucleotide sequence of the R.meliloti nitrogenase reductase (nifH) gene. Nucleic Acids Res. 1981 Nov 11;9(21):5711–5723. doi: 10.1093/nar/9.21.5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinman J. J., Fellows F. F., Gresshoff P. M., Shine J., Scott K. F. Structural analysis of the genes encoding the molybdenum-iron protein of nitrogenase in the Parasponia rhizobium strain ANU289. Nucleic Acids Res. 1984 Nov 26;12(22):8329–8344. doi: 10.1093/nar/12.22.8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston M. F., Kotake S., Davis L. C. Interaction of nitrogenase with nucleotide analogs of ATP and ADP and the effect of metal ions on ADP inhibition. Arch Biochem Biophys. 1983 Sep;225(2):809–817. doi: 10.1016/0003-9861(83)90093-0. [DOI] [PubMed] [Google Scholar]
- Yun A. C., Szalay A. A. Structural genes of dinitrogenase and dinitrogenase reductase are transcribed from two separate promoters in the broad host range cowpea Rhizobium strain IRc78. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7358–7362. doi: 10.1073/pnas.81.23.7358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumft W. G., Mortensson L. E. Evidence for a catalytic-centre heterogeneity of molybdoferredoxin from Clostridium pasteurianum. Eur J Biochem. 1973 Jun 15;35(3):401–409. doi: 10.1111/j.1432-1033.1973.tb02852.x. [DOI] [PubMed] [Google Scholar]